Dry eye disease (DED) affects more than 14% of the elderly population causing decrease of quality of life, high costs and vision impairment. Current treatments for DED aim at lubricating and controlling inflammation of the ocular surface. Development of novel therapies targeting different pathogenic mechanisms is sought-after. The aim of this study, published in British Medical Journal, was to evaluate safety and efficacy of recombinant human nerve growth factor (rhNGF) eye drops in patients with DED.
Nerve growth factor (NGF) plays a crucial role in modulating function of central and  peripheral nervous systems, endocrine, immune and visual system. it’s discovery in the 1950s has led to increasing experimental and clinical evidence as an essential factor for the trophism, sensitivity and healing of the cornea and the conjunctiva.
peripheral nervous systems, endocrine, immune and visual system. it’s discovery in the 1950s has led to increasing experimental and clinical evidence as an essential factor for the trophism, sensitivity and healing of the cornea and the conjunctiva.
In patients with neurotrophic keratitis and autoimmune corneal ulcers, early clinical studies have shown safe and efficacious topical treatments with murine NGF (mNGF) eye drops. Both human and animal studies indicate that exogenously administered NGF stimulates ocular surface wound healing and sensitivity and increases tear production and conjunctival goblet cells density. All of these factors indicate that NGF may be a potentially beneficial treatment for dry eye disease (DED).
Currently, the most common treatments lubricate the exposed epithelia with tear substitutes and control ocular surface inflammation with immunosuppressive drugs and steroids. This study seeks to explore novel therapeutic approaches that target different mechanisms of DED, such as impairment of ocular surface sensitivity and epithelial damage.
A phase 1 clinical trial tested rhNGF eye-drop formulation successfully, showing a good safety and tolerability profile. In July 2017, European Medicine Agency licensed full market authorization for the use of rhNGF (Cenegermin) eye drops for the treatment of moderate and severe neurotrophic keratitin (NK). In August, 2018, the Food and Drug Administration approved Oxervate (cenegermin) for treatment of neurotrophic keratitis. This represents the first US approval of an agent utilizing recombinant human nerve growth factor (rhNGF). The drug had received Orphan Drug Designation, Fast Track Status, Breakthrough Therapy Designation, and Priority Review from the FDA before its full marketing approval. Read more here.
In this study, researchers present the results of the first European phase II clinical trial using rhNGF eye drops in patients with moderate to severe DED.
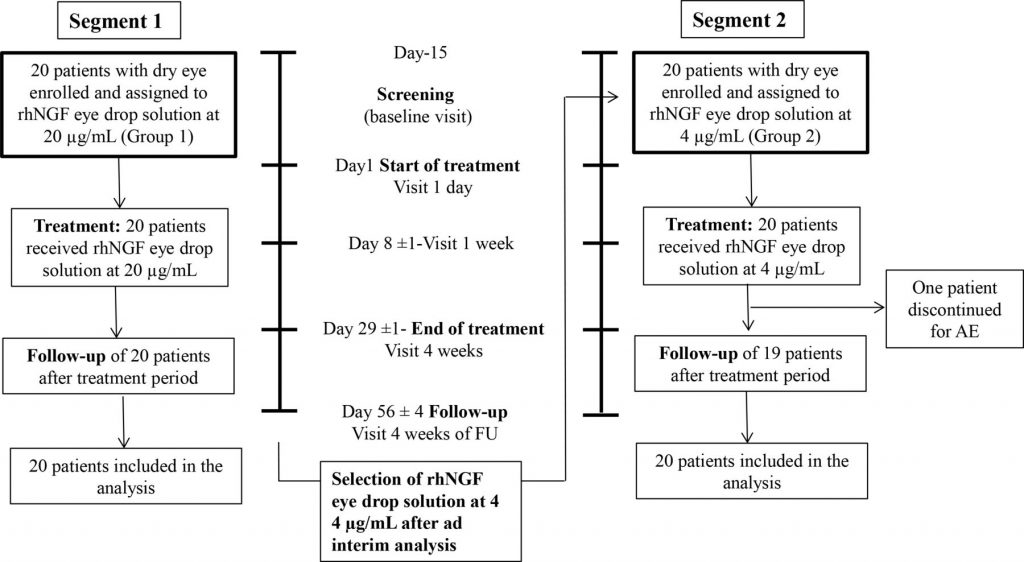
Methods / Forty consecutive patients with moderate to severe DED were included in a phase IIa, prospective, open label, multiple-dose, clinical trial to receive rhNGF eye drops at 20 µg/mL (Group 1: G1) or at 4 µg/mL (Group 2: G2) concentrations, two times a day in both eyes for 28 days (NCT02101281). The primary outcomes measures were treatment-emerged adverse events (AE), Symptoms Assessment in Dry Eye (SANDE) scale, ocular surface staining and Schirmer test.
Results / Of 40 included patients, 39 completed the trial. Both tested rhNGF eye drop concentrations were safe and well tolerated. Twenty-nine patients experienced at least one AE (14 in G1 and 15 in G2), of which 11 had at least 1 related AE (8 in G1 and 3 in G2). Both frequency and severity of DED symptoms and ocular surface damage showed significant improvement in both groups, while tear function improved only in G1.
- Both doses of rhNGF eye drops improved ocular surface damage at the end of treatment period.
- These effects were associated with a significant improvement of tear film production.
- A long-lasting effect of rhNGF eye drop treatments was observed at the follow-up visit (4 weeks after the end of treatment), as compared with baseline values.
- Comparison of clinical effects of the two rhNGF doses showed a significant improvement of NEI conjunctival total score at the end of treatment period.
Conclusions / This is the first study that demonstrates the safety and efficacy of topically applied rhNGF in patients with DED. Specifically, topical ophthalmic formulation of rhNGF at both 4 µg/mL and 20 µg/mL revealed no local or systemic safety concerns and showed good tolerability in patients with moderate to severe DED. The majority of AEs were mild in severity and resolved without treatment, and no patients were discontinued for AEs related to rhNGF eye drop treatment. The results are in line with the safety results observed in the phase II clinical trials performed in patients with neurotrophic keratoconjunctivitis treated with rhNGF eye drop at concentrations of 20 µg/mL or 10 µg/mL for 8 weeks. The results of this studies showed a good safety profile of treatment with rhNGF eye drop at 20 µg/mL, with eye pain being the most frequent treatment-related AE and no new AEs reported as compared with other studies.
The data of this study indicates that rhNGF eye drops in both doses is safe and effective in improving symptoms and signs of DED. Randomized clinical trials are ongoing to confirm the therapeutic benefit of rhNGF in DED.
Click Here for Full Text Study
Source: Sacchetti M, Lambiase A, Schmidl D, et al Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study. British Journal of Ophthalmology. Published Online First: 03 April 2019. doi: 10.1136/bjophthalmol-2018-312470