A Grim Reality: The Prostate Cancer Burden
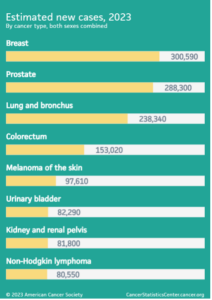
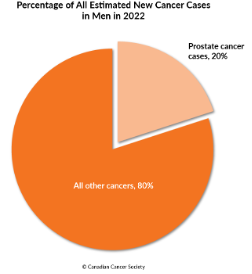
Prostate cancer remains a critical public health issue, affecting the lives of men across the globe. In the United States alone, 2023 estimates show about 288,300 new cases and approximately 34,700 deaths from prostate cancer. The risk is higher among older and non-Hispanic Black men, with about 1 in 8 men expected to receive a prostate cancer diagnosis during their lifetime [Prostate Cancer Statistics. American Cancer Society, 2023. (Accesses August 30, 2023)]. Canada echoes these troubling statistics, with an estimated 24,600 new cases in 2022 and 4,600 deaths (Figure 1.). The scale of the problem is immense and mandates urgent interventions [Prostate Cancer Statistics. Canadian Cancer Society, 2022. (Accessed August 30, 2023)].
Figure 1. On the left: Projected cancer case counts in the USA for 2023, encompassing both genders. On the right: Proportion of anticipated new male cancer diagnoses in Canada for 2022.
Despite improvements in early detection and treatment methods that led to a decline in prostate cancer death rates, the pace of this decline has slowed in recent years. This troubling trend underscores the pressing need for novel approaches to both the treatment and management of prostate cancer.
The Evolution of MAG-EPA: From Inflammation to Dietary Adjuvant to Cancer Treatment
The Inception: Resolving Inflammation
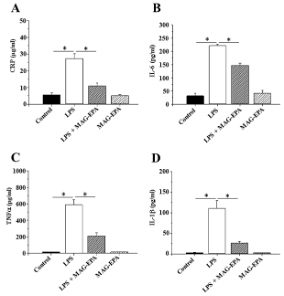
The journey of MAG-EPA (Eicosapentaenoic Acid Monoacylglyceride) began when researchers first observed its potential for mitigating inflammation in pre-clinical studies. Utilizing ex vivo models of human peripheral blood mononuclear cells, the early studies revealed that MAG-EPA was effective in dampening inflammatory pathways. It specifically downregulated key inflammatory markers, including IL-1β, IL-6, and TNFα (Figure 2.), pointing to its potential therapeutic role in resolving inflammation in various disorders such as autoimmune diseases, allergies, and even cancer (Morin C, et al. Eur J Pharmacol. 2017 Jul 15;807:205-211).
Figure 2. Effect of MAG-EPA on pro-inflammatory mediator levels in LPS-treated PBMC. A: Reactive protein C (CRP) B: IL-6, C: TNFα and D: IL-1β levels were assessed in culture media derived from Control, LPS-treated, LPS + MAG-EPA-treated and MAG-EPA-treated PBMC using specific ELISA as described in the Methods section (n =6, * P≤0.05).
The Revelation: Targeting Colorectal Cancer Cells
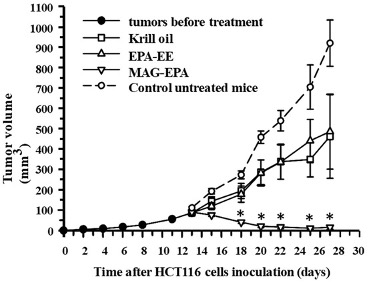
The story took an interesting turn when MAG-EPA demonstrated its potency against human colorectal cancer cells, much more so than regular fish oil and Krill oil (Figure 3.). In vitro and in vivo mouse models showed that the compound effectively reduced cell proliferation and induced apoptosis. Moreover, MAG-EPA treatments suppressed pathways crucial to cancer cell survival and growth, such as EGFR and VEGFR activation. This further research underlined its therapeutic potential not just in resolving inflammation but also in actively countering cancer cell growth and proliferation (Morin C, et al. Mar Drugs. 2017 Sep 4;15(9):283).
Figure 3. Effect of EPA-EE and Krill oil treatment on tumor growth in the HCT116 xenograft nude mice model. Tumor growth (mm3) as a function of time (day) was measured after subcutaneous injection of 1 × 106 HCT116 cells in EPA-EE (618 mg/kg), Krill oil (618 mg/kg) and MAG-EPA (618 mg/kg)-treated mice. Compounds were administered orally daily dose of 618 mg/kg total omega-3 from day 13 to day 27. Results represent the mean volume ± SEM (n = 6 per group, * p < 0.05).
The Gateway: Probing Prostate Tumor Vascularity
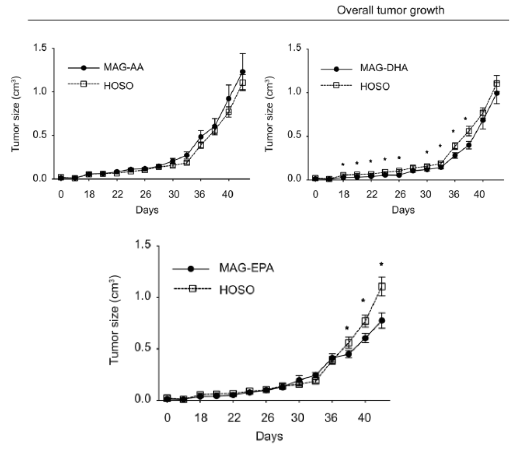
With mounting evidence supporting MAG-EPA’s anticancer potential, the research shifted toward understanding its role in prostate cancer. In pre-clinical studies, MAG-EPA supplementation significantly reduced prostate tumor growth compared to the HOSO control (Figure 4.). The tumors from MAG-EPA-treated mice showcased distinct mRNA patterns, leaning heavily towards genes related to angiogenesis and vasculature. Further analysis confirmed MAG-EPA’s influence on these vascular pathways in TRAMP-C2 prostate tumors. Notably, these tumors had fewer and smaller blood vessels, emphasizing MAG-EPA’s potential to modify tumor vascularization (Gevariya N, et al. Mol Cancer Res. 2021 Mar;19(3):516-527).
Figure 4. Effects of purified fatty acid supplements on TRAMP-C2 tumors in vivo. Mice were supplemented with either MAG-AA, -EPA, -DHA or HOSO until animal sacrifice (endpoint 2 cm3 tumor size). Curves of overall tumor growth. Asterisk represents P < 0.05 from a linear mixed model with repeated measures test, comparing each fatty acid treatment with the HOSO group (HOSO n = 11, AA n = 10, EPA n = 11, DHA n = 12; four mice were sacrificed before endpoint due to ulceration).
Building Upon Foundational Insights: The RCT-EPA Trial
Drawing on the accumulating pre-clinical evidence of MAG-EPA’s potential in cancer therapeutics, the RCT-EPA trial was conceived. As a phase IIb, randomized, double-blind, placebo-controlled study involving 130 men, this study is positioned to offer a holistic examination of MAG-EPA’s implications in prostate cancer. By focusing on patients about to undergo radical prostatectomy, especially those with a Gleason score ≥ 7, the trial is strategically aligned to discern the possible impacts of MAG-EPA supplementation on cancer cell proliferation, inflammation modulation, and the broader spectrum of patient quality of life (Guertin MH, et al. BMC Cancer. 2018 Jan 10;18(1):64).
While the comprehensive results of the RCT-EPA trial are still on the horizon, four foundational publications have already surfaced, offering glimpses into the early findings. These inaugural reports foreshadow the forthcoming, more detailed examinations set to address the study’s primary and secondary objectives. As the broader scientific community and stakeholders with vested interests anticipate these in-depth revelations, the subsequent sections embark on an analytical journey through these initial discoveries, shedding light on the varied therapeutic avenues of MAG-EPA, spanning from molecular intricacies to direct clinical implications.
Insights from the RCT-EPA Trial: Preliminary Findings from Early Publications
RCT-EPA 001: The Vasculature Dynamics in Prostate Tumors Post MAG-EPA Supplementation
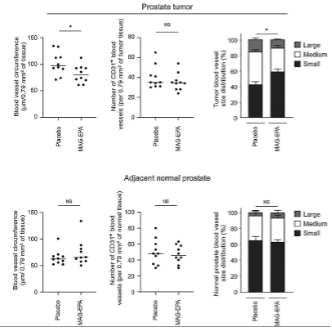
From the breadth of the RCT-EPA trial, the inaugural publication illuminates MAG-EPA’s role in human prostate cancer. It centered on men set for radical prostatectomy, given either 3g/d of MAG-EPA or a placebo over 8 weeks. As depicted in Figure 5, while both groups had comparable vessel counts, the MAG-EPA recipients showed predominantly smaller vessels in their tumors. This was exclusive to the tumor, highlighting MAG-EPA’s targeted impact. A significant observation was the elevated EPA in the RBC membranes and tumor samples of the MAG-EPA group, underlining its influence on tumor vasculature (Gevariya N, et al. Mol Cancer Res. 2021 Mar;19(3):516-527).
Figure 5. Effects of MAG-EPA or placebo on tumor vasculature-related phenotype in human prostate tumor tissues. Vascularization was analyzed by IHC in prostate tumor of men undergoing radical prostatectomy after a 4–10 weeks supplementation with either MAG-EPA or placebo (HOSO). Prostate tissue at surgery for placebo- (n = 10) and MAG-EPA- (n = 10) supplemented groups was stained for CD31 using a specific antibody. CD31 histological analysis were performed for prostate tumors (B) and normal adjacent prostate tissue (C). *, P < 0.05.
RCT-EPA 002: The Interplay of Gut Microbiome and Prostate Tumor Growth
Delving into the complexities of the gut microbiome and its potential interplay with prostate cancer progression, this early publication from the RCT-EPA trial unravels a fascinating nexus. Observations spotlighted the instrumental role of gut microbiota in modulating immune-centric cancer therapies. A pivotal discovery was the correlation between reduced gut microbiota diversity and advancing prostate tumor stages. MAG-EPA’s introduction as a dietary component appeared to modulate tumor growth, particularly noted by a decline in fecal butyric acid levels. This aspect underscores the vital role of dietary shifts, especially the introduction of omega-3 fatty acids, in potentially curbing prostate tumor progression (Jalal Laaraj, et al. Cancer Immunol Res 1 January 2022; 10 (1_Supplement): P001).
RCT-EPA 003: Impact on Surgical Blood Outcomes
Given the perennial concerns surrounding the possible side effects of omega-3 fatty acids in the perioperative scenario, this segment of the RCT-EPA trial offers comforting insights. Findings reaffirm the safety of MAG-EPA supplementation, as it presented no significant amplification of perioperative bleeding risks. Alongside, its potential merits in decelerating prostate cancer progression accentuate the dual boon of omega-3 supplementation – therapeutic efficacy aligned with surgical safety (Fradet S, et al. Clin Nutr ESPEN. 2022 Feb;47:221-226).
RCT-EPA 004: A Glimpse into Quality of Life after Radical Prostatectomy
Patient well-being post-surgery is undeniably paramount. An early deep dive from the RCT-EPA study evaluates MAG-EPA’s impact on the quality of life post-radical prostatectomy. While immediate post-operative observations seemed indistinct, a revelation emerged in the 12-month follow-up: those on the MAG-EPA regimen showed noticeable improvements in urinary irritation function scores. This casts a promising light on prolonged MAG-EPA supplementation’s potential to amplify post-operative quality of life, especially concerning urinary functions (Moussa H, et al. Nutrients. 2023 Mar 11;15(6):1369).
Conclusion: An Evolving Paradigm in Prostate Cancer Management
MAG-EPA’s evolution, from its initial identification as an inflammation resolution compound to its emerging role in prostate cancer therapeutics, presents a notable progression in oncology research. Early data from the RCT-EPA trial has provided foundational insights into the multifaceted benefits of MAG-EPA, evidenced not solely at the cellular and molecular interfaces but also through observable improvements in patient post-operative recovery and quality of life metrics. As we anticipate more detailed outcomes from the extensive RCT-EPA study, present results underscore the potential of MAG-EPA dietary supplement as an innovative adjunct in prostate cancer treatment paradigms. The medical community, advancing towards a patient-oriented approach, should consider MAG-EPA beyond its conventional therapeutic scope. It offers a promising avenue, potentially enhancing the therapeutic journey for those diagnosed with prostate cancer.