Although the lungs are a common target for COVID-19’s cytokine storm, so are the kidneys, making the 1-in-4 U.S. adults with diabetes resulting in diabetic kidney disease at increased risk for virus mortality.
But why are the kidneys so attractive to the coronavirus?
Recently published in Kidney International, a national research team made up of kidney clinicians, bioinformaticians, a molecular biologist, pathologist and virologist found that a protein on the surface of some kidney cells, called angiotensin-converting enzyme 2 (ACE2), is the primary COVID-19 entry receptor and aids in the activation of its uncontrolled immune response.
It also is responsible for the virus’ duplication, leaving patients sicker, longer. Since higher levels of ACE2 expression on cells correlates with higher risk of serious COVID-19 illness, Matthias Kretzler, M.D., a study author and nephrologist at Michigan Medicine, sought out to learn more about which kidney cells create elevated levels of this protein and why, as well as if the molecular process of the vulnerable cells is similar to those in patients with COVID-19.
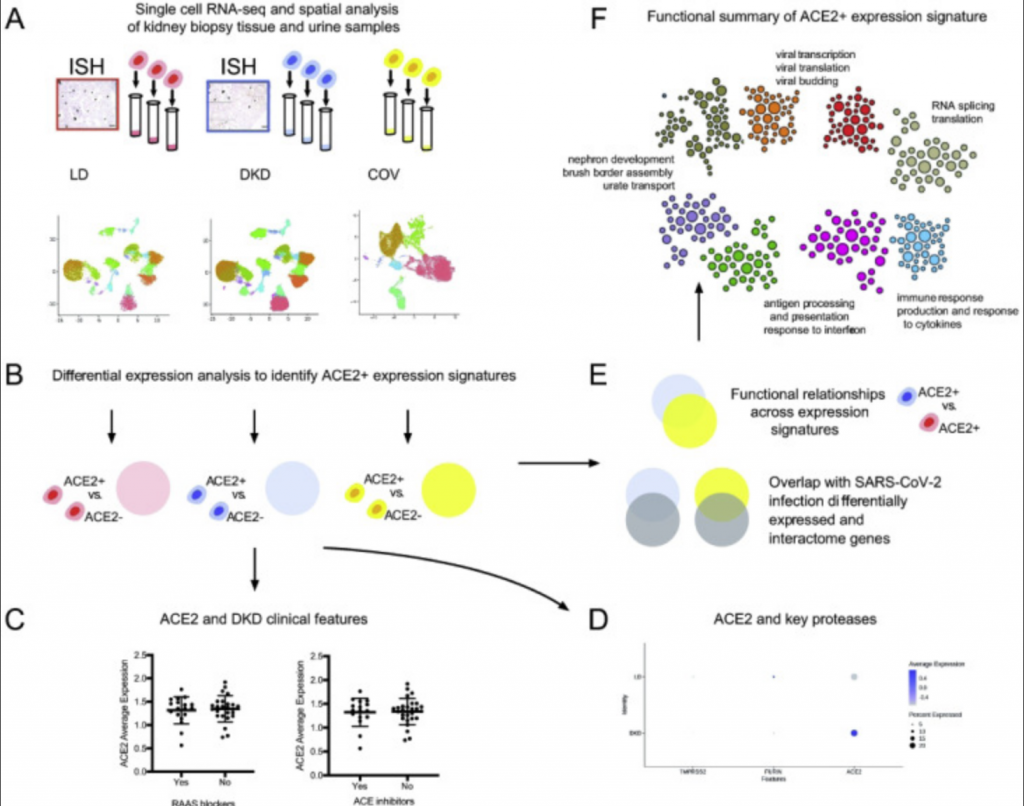
Using machine learning technology developed by study author Olga Troyanskaya, Ph.D., from Princeton University, the researchers were able to identify and categorize groups of genes that produced higher ACE2 expression levels in three different subject groups: healthy, living kidney donors (18 participants), those with diabetic kidney disease (44 participants) and those hospitalized with COVID-19 (13 participants.)
After analyzing more than 110,000 different cells in the three groups, Kretzler and the team identified networks of molecules that result in higher levels of ACE2.
“Being able to characterize these molecular processes may help scientists quickly identify and develop therapies to lessen the risk of serious illness for patients, or even prevent COVID-19 infection from damaging the kidney,” Kretzler says.
The groups shared a few molecular similarities, but one would become the focus of the researchers: ACE2 was predominantly expressed in cells that also expressed markers of specialized transport epithelial cells in the proximal tubules. This area of the kidney is responsible for reabsorbing nutrients during the kidney’s filtration process.
Using machine learning technology
Using kidney biopsies from the healthy kidneys and those with diabetic kidney disease, and kidney cells retrieved from the urine samples of COVID-19 patients, the machine learning technology allowed the research team to pinpoint in what kidney cells ACE2 is found and what characteristics these cells have.
Cells that express the virus receptor, ACE2, were found to be “locked and loaded” for the virus, meaning many other proteins are found with ACE2 which interact with viruses during infection.
This wasn’t only true in the COVID-19 infected patients, but also in kidneys from patients with diabetes. When comparing the kidney cells of COVID-19 patients with those with diabetic kidney disease, similar molecular processes were activated in both that would trigger severe COVID-19 illness.
“Diabetic kidney disease, by nature, primes kidney cells in a way that can make them vulnerable to COVID-19,” Kretzler says. “In conjunction with COVID-19 and its inflammatory nature, serious kidney damage can occur.
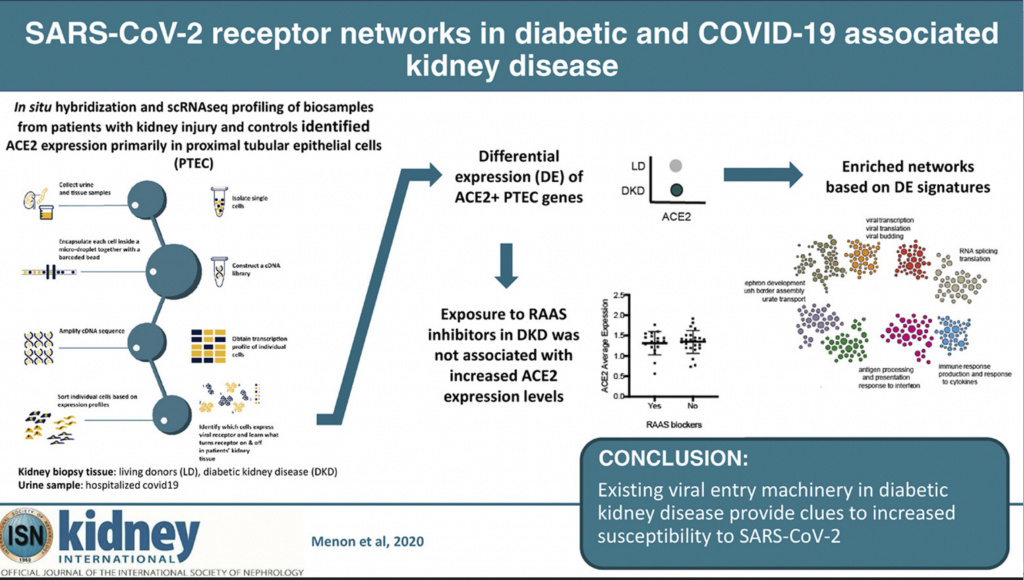
To understand how the kidney may be impacted by COVID-19 researchers performed spatial, systems, and clinical association analyses of ACE2 and other SARS-CoV-2 host factors in kidney biopsies from living donors (LD) and diabetic kidney disease (DKD) patients and kidney cells isolated from the urine of hospitalized COVID-19 patients (COV).
What is Known and Is Still Unknown
Discovering the importance of the proximal tubules epithelial cells in its relation to the severity of COVID-19 illness opens a door for novel therapeutics to address COVID-19 and its related kidney injury.
“We weren’t sure before this study if medications commonly used to treat hypertension and diabetic kidney disease increase the risk of COVID-19 infection. There was a serious concern from colleagues and my patients about how these medications affect ACE2 in the kidney,” Kretzler says.
Now, the team can confidently conclude these medications won’t harm those with diabetic kidney disease, providing reassurance for patients to continue to take these live saving medicines. However, further studies need to look at a population that has both diabetic kidney disease and COVID-19. Kretzler confirms these are underway.
“To help in the global research response to COVID-19, we made our data available to scientists around the world so that they can use the information from our patients to help identify novel ways to treat patients in the pandemic,” Kretzler says. “Our team is focused now on learning how treatments given to COVID-19 patients affect kidney cells, so we can offer the best medications to patients with COVID-19 and kidney disease in the ongoing pandemic.”
Conclusion/ With the pandemic spread of SARS-CoV-2 and the increased morbidity and mortality of COVID-19-associated disease in patients with diabetes and kidney disease, it is imperative to define the underlying mechanisms that would promote rapid development of risk reduction strategies. The work identifies the regulation and associated cellular machinery of ACE2 and associated SARS-CoV-2 co-receptors in PTEC in kidney health, metabolic and viral disease. The SARS-CoV-2 receptor associated networks are now available (hb.flatironinstitute.org/covid-kidneyhb.flatironinstitute.org/covid-kidney) to seed further research into urgently needed therapeutic strategies for COVID-19.
Click Here for Full Text Study