The following is a summary of a human-subject study on herring roe oil with clinical meaningful results for supporting patients with mild psoriasis vulgaris. Following the summary is an interview with study co-author, Hogne Hallaraker, founder and Chief Science Officer of Arctic Bioscience.
In recent small but significant human-subject study, researchers explored the effect of Omega-3 fatty acids, specifically from herring roe oil as a dietary supplement, for psoriasis vulgaris. After previously inconclusive study results, this new research showed promising results for decreased symptoms and clinically meaningful improvements in quality of life for individuals with psoriasis.
When diagnosing psoriasis, the severity is categorized based on the extend of the body surface area (BSA) and the Psoriasis Area Severity Index (PASI). Though there are a significant number of treatments for patients with PASI scores above 10, advances in therapy for those with PASI scores of less than 10 and a BSA lower than 10 have been limited. Previously, a systematic review looked at 12 Omega-3 studies and found that 10 of those studies had a duration of treatment of less than 26 weeks. Also, the ratio of eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) was typically 1:1, or contained more EPA than DHA.

In this latest study, the efficacy of herring roe oil was researched in a randomized, double-blind placebo-controlled environment on patients with a baseline PASI score of less than 10. And the duration of the study lasted for 26 weeks. All study participants were invited to continue in an open-label 39-week active treatment period.
The study included patients with stable psoriasis for at least 6 months and PASI scores below 10. Patients on regular local psoriasis topical medication for more than 2 months before the study start continued on treatment. And, there were no limitations on use of non-medicated creams. Each HRO capsule contained 292 mg polyunsaturated fatty acids, 22% EPA and 66% DHA, where 35% of both were bound to phospholipids including phosphatidylcholine. The total dose of DHA and EPA was 2.6g and the total lipid was 5.9g. Patients discontinued cod-liver oil, Omega-3 and choline prior to the study. Vitamin D, omega-3 and choline were prohibited during the study. Diet did not change, including fish intake, alcoholic intake was prohibited.
The herring roe oil study reported the following results:
1. In the HRO-HRO treatment group (n =28) reductions from baseline to week 26 in mean patient reported outcome measures (PROMs) ranged from 25-41%.
2. Corresponding reductions in the placebo-HRO group (n=30) ranged from 5-to-23%)
3. The proportion of patients achieving a Physicians Static Global Assessment (PSGA) of 0 or 1 (clear or almost clear) was 39.7% at 65 weeks.
4. All patients had a PSGA score of >/- 2 and >/- 4 at inclusion and after 65 weeks, no patient had a score of higher than 3. 5. In total, 46.6% of patients had a reduction in their PSGA score.
5. In the entire 15 months study, BSA mean values dropped by 46% in the HRO-HRO group and 41% in the placebo-HRO group. Overall drop in all participants was 44%.
6. Patients also reported reduction of skin disease activity, pruritus, skin pain and singeing in the open label extension group (measured in mean visual analogue scale, VAS).
7. Participants reported the following improvements: 42% skin disease activity, 46% pruritus and 52% singeing over the 65 weeks. 8. The mean Dermatology Life Quality Index (DLQI) scores and changes in scores was 54% in HRO-HRO group and 47% in placebo-HRO group.
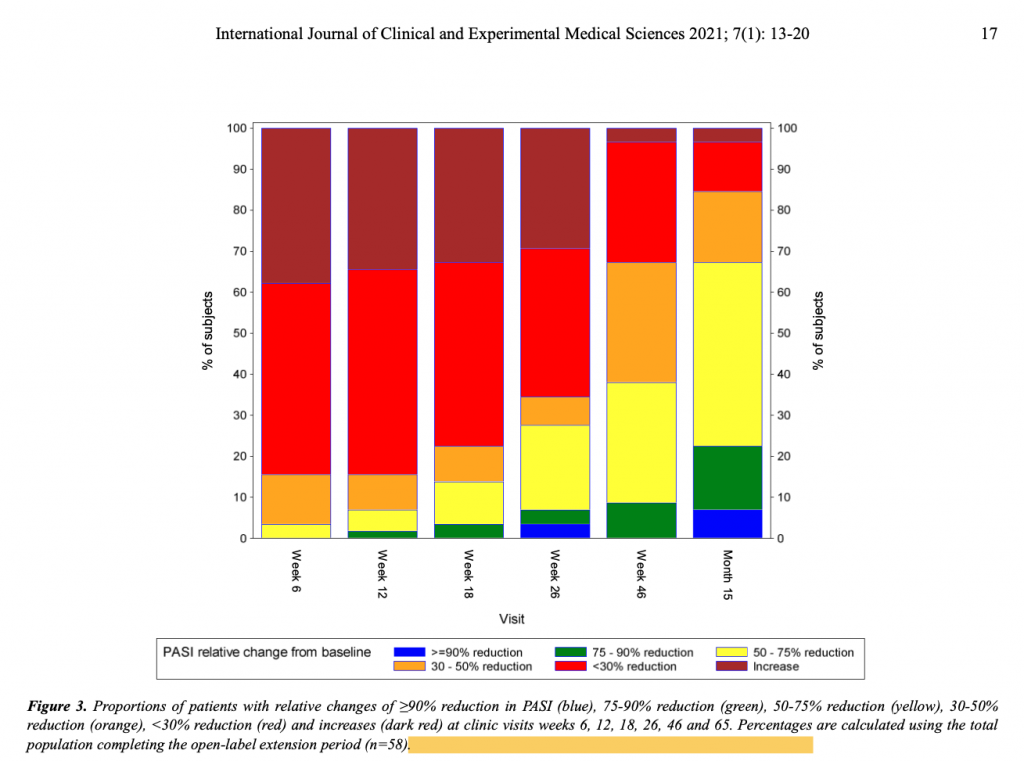
Conclusion/ The similarity of disease improvements as measured by the investigator outcome measures PASI, PSGA and BSA and the patient outcome measures (VAS and DLQI), as well as their magnitude, strengthens the assumption that HRO improves psoriasis when administered for at least 26 weeks and has demonstrated a good safety profile over 65 weeks. Thus, HRO may be a promising option for non-severe psoriasis, especially when other treatments have failed. The results of this study should be confirmed in a larger human clinical trial.
Interview with Hogne Hallaraker, study co-author, founder and Chief Science Officer of Arctic Bioscience.
Q: According to the World Psoriasis Consortium and National Psoriasis Foundation, psoriasis affects 8 million people in the United States and 125 million people globally. How might this study make a difference to these millions of people?
Hogne Hallaraker: Up to 90% of psoriasis patients fall in the mild to moderate psoriasis segment, while 10% are in the severe psoriasis stage. Therefore, 7.2 million U.S. patients fall in the mild to moderate condition. There have been significant advances in the treatment of more severe psoriasis such as the injectable biologics and other pharmaceutical regimens. These drugs, while effective, are also associated with side effect risk and the risk/reward balance in treatment options must always be considered. In many cases, these treatments block a specific immune response mechanism, as part of their mode of action and efficacy, and are contraindicated for some patients.
On the other hand, there has been little advancement in the treatment of mild to moderate psoriasis beyond the traditional steroid creams and other topical interventions.
The results of this dietary intervention pilot study will form the basis of a new investigational medicinal product within the company where the goal is to develop a new and innovative API targeting mild to moderate psoriasis. We are therefore currently in the process of setting up a larger phase IIb clinical study that hopefully will give us more detailed insight into both dose/response and mechanisms of action in the near future.
Q: Beyond psoriasis, what are the other potential health properties of HRO that clinicians may find useful in their practice?
HH: Given the outcome of our published pilot trial and related research we think researching other auto-immune indications would be a natural step forward.
Q: While the current clinical research in Psoriasis has been focussed on development of a potential API application, are there currently opportunities for utilizing the Herring Roe Extract in the nutraceutical category for maintenance of good health and healthy aging?
HH: Yes, Arctic Bioscience has established a portfolio of Romega® Herring Caviar Oil products available for supplement applications in the nutraceutical arena. These products differ from the API candidate through a different composition and content of omega-3 phospholipids and a lower dosage. However, the omega-3 phospholipids in Romega deliver both enhanced bioavailability compared to standard TG fish oils (reference: Cook et al 2016) and are found in a 3:1 DHA to EPA ratio where 80% of the Omega-3 bound phospholipids are in the form of phosphatidylcholine, an essential ingredient for neurological development. The supplement line Romega Herring Caviar Oils is available either as bulk oil or as bulk 500 mg capsules.
For further information, www.herringcaviarextracts.com, www.arctic-bioscience.com; Rick Pope – SVP B2B Nutraceuticals, Arctic Bioscience A.S.+1 902-292-1657. Arctic Bioscience recently changed their company name. More here.