Though we know about the importance of sensory neurons in gut-brain communication and how this relates to satiety and blood glucose regulation, just how these cells actually participate in the regulation of feeding and blood glucose levels was an unknown. New mice research, published in Cell Metabolism, sheds light on how the vagus nerve, gut and brain communicate with each other in order to adapt satiety and blood sugar levels during food consumption. In other words, the vagus nerve cells detect what we eat and respond to fulfill opposing tasks of satiety and blood sugar regulation.
Researchers from the Max Planck Institute for Metabolism Research in Cologne, the Cluster of Excellence for Ageing Research CECAD at the University of Cologne and the University Hospital Cologne looked at the functions of the different nerve cells in the control center of the vagus nerve, and discovered something very surprising.
Although the nerve cells are located in the same control center, they innervate different regions of the gut and also differentially control satiety and blood sugar levels. This discovery could play an important role in how we understand the gut-brain axis for obesity, diabetes and other metabolic health disorders.
As they explain, when one consumes food, information about the ingested food is transmitted from the gastrointestinal tract to the brain in order to adapt feelings of hunger and satiety. Based on this information, the brain decides, for example, whether we continue or stop eating. In addition, one’s blood sugar level adapt through the brain. The vagus nerve, which extends from the brain all the way down to the gastrointestinal tract, plays an essential role in this communication.
In the control center of the vagus nerve, the nodose ganglion, various nerve cells are situated, some of which innervate the stomach, while others innervate the intestine. Some of these nerve cells detect mechanical stimuli in the different organs, such as stomach stretch during feeding, while others detect chemical signals, such as nutrients from the food that we consume. But what roles these different nerve cells play in transmitting information from the gut to the brain, and how their activity contributes to adaptations of feeding behavior and blood sugar levels had remained largely unclear.
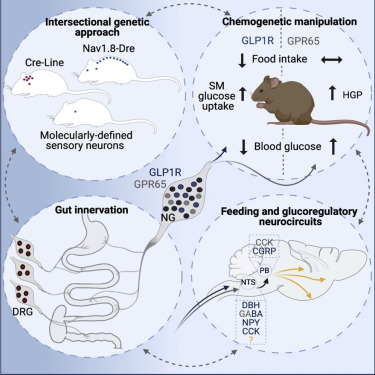
The researchers reconstructed the gut innervation patterns of numerous molecularly defined vagal and spinal afferents and identify their downstream brain targets in mice.
“To investigate the function of the nerve cells in the nodose ganglion, we developed a genetic approach that enables us to visualize the different nerve cells and manipulate their activity in mice. This allowed us to analyze which nerve cells innervate which organ, pointing to what kind of signals they detect in the gut,” says study leader Henning Fenselau. “It also allowed us to specifically switch on and off the different types of nerve cells to analyze their precise function.
Different Food Activates Different Nerve Cells
In their studies, the researchers focused primarily on two types of nerve cells of the nodose ganglion, which is just one millimeter in size.
“One of these cell types detects stomach stretch, and activation of these nerve cells causes mice to eat significantly less,” Fenselau explains. “We identified that activity of these nerve cells is key for transmitting appetite-inhibiting signals to the brain and also decreasing blood sugar levels.”
The second group of nerve cells primarily innervates the intestine.
“This group of nerve cells senses chemical signals from our food. However, their activity is not necessary for feeding regulation. Instead, activation of these cells increases our blood sugar level,” says Fenselau. Thus, these two types of nerve cells in the control center of the vagus nerve fulfill very different functions
“The reaction of our brain during food consumption is probably an interplay of these two nerve cell types,” Fenselau explains. “Food with a lot of volume stretches our stomach, and activates the nerve cell types innervating this organ. At a certain point, their activation promotes satiety and hence halts further food intake, and at the same time coordinates the adaptations of blood sugar levels. Food with a high nutrient density tends to activate the nerve cells in the intestine. Their activation increases blood glucose levels by coordinating the release of the body’s own glucose, but they do not halt further food intake.”
In summary, they discovered that distinct gut-innervating sensory neurons differentially control feeding and glucoregulatory neurocircuits, which may provide specific targets for metabolic control.
CONCLUSION/ Given the recent identification of genetically distinct vagal and spinal sensory neurons that innervate different organs of the GI tract (Bai et al., 2019; Hockley et al., 2019), this intersectional approach, coupled with existing or newly generated transgenic mouse lines, provides a mean for future functional interrogation of these neurons in gut-brain communication in normal and disease states. The discovery of the different functions of these two types of nerve cells could play a crucial role in developing new therapeutic strategies against obesity and diabetes.
Click Here for Full Text Study