“The brain and the gut speak the same language” (1) Ethan Russo, MD, Renown Cannabinoid Scientist

The endocannabinoid system (ECS) is a network of receptors and enzymes that are spread throughout the body. This system can interact with one’s own cannabinoids (endogenous or endocannabinoids) because of substances found in nature called phytocannabinoids, which are present in Cannabis Sativa – hemp and marijuana – but also in other plants including black pepper, clove, ginseng, echinacea, and kava, as well as a range of developed and developing pharmaceuticals. This paper will discuss the role of the ECS and the gut, but before we get to that, I’ll review what is known thus far about the ECS.
The major components of the ECS are:
- 2 endogenous cannabinoids: Anandamide (AEA) and 2-arachidonoyl glycerol (2-AG)
- 2 primary receptors: CB1 and CB2
- 2 synthesizing enzymes: Diacylglycerol lipase and N-acyl-phosphatidylethanolamine-phospholipase D
- 2 degrading enzymes: Fatty acid amide hydrolase (FAAH), which breaks down anandamide, and monoacylglycerol lipase (MAGL), which breaks down 2-AG
As knowledge of the ECS is expanding rapidly at this time, other components of the system are being discovered, mostly new receptors. Most natural compounds or drugs that interact with the ECS either act on a receptor or a degrading enzyme, though other mechanisms are also possible. The receptors include GPR55, GPR18, TRPV1 (the vanilloid or capsaicin receptor), and a possible CB3 receptor. Other endogenous ligands include Virodhamine, NADA (N-Arachidonoyl dopamine), and possibly Noladin (2-Arachidonyl glyceryl ether, 2AGE).
The Endocannabinoid System and the Gut
Both of the major receptors of the endocannabinoid system, along with their synthesizing and degrading enzymes, are found in your digestive system. The ECS serves multiple roles in the gut depending on the type of receptor, the location of the receptor, and the overall state of digestive system health. Some of the major functions of the ECS in the gut are the following:
Modulates Inflammation
The endocannabinoid system seems to play a key role in protecting the gut from inflammation. Both CB1 and CB2 receptors will help to modulate an inflammatory response when stimulated. If there is more active inflammation in the gut, the body seems to recruit more CB2 receptors to regulate pain, inflammatory response, and motility.(2) See below in Figure 1. In fact, the current body of evidence indicates that the overall “tone” of the ECS is increased in the presence of chronic inflammation in the intestines. In addition to CB2 recruitment, expression of CB1 receptors and TRVP1 activity may also be increased. Studies of patients with ulcerative colitis, diverticulitis, and celiac disease have all shown elevated levels of anandamide.(3) Collectively, these mechanisms are theorized to be an attempt by the body to safeguard against inflammatory damage.
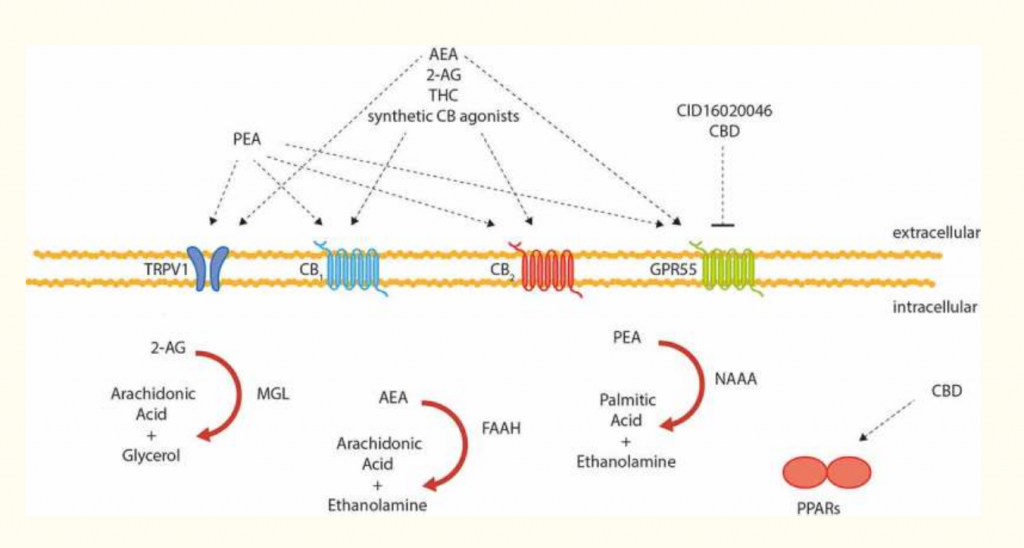
Currently several phytocannabinoids and cannabinoid drugs are being studied as treatments for inflammatory bowel disease and related conditions. Synthetic CB1 agonists such as ACEA and HU-210 (which have similar or stronger action than THC without the potential impairment) and JWH-133 (a potent CB2 agonist) are all potential drug candidates.(4) Cannabigerol (CBG), which can be derived from either hemp or marijuana, has shown promise in animal models of colitis. (5) Beta-carophyllene – a sesquiterpenoid phytocannabinoid found in cannabis but also in many other plants – is a selective CB2 agonist that is being looked at in a variety of GI conditions from gastritis to colitis.
Helps to Regulate Motility
Cannabis compounds have been described in historical medical texts going back centuries as treatments for conditions like diarrhea and cramping. The best understood mechanism is activation of the CB1 receptor, which has a strong impact on decreasing motility. CB1 receptors are highly expressed throughout the mesenteric nervous system. Their activation leads to an inhibition of acetylcholine (ACH) release. This, in turn, leads to slowed peristalsis and reduced smooth muscle contraction, and accounts for some of the better-known uses of THC (the dominant cannabinoid in marijuana and a strong CB1 agonist) for GI discomfort – including calming nausea, slowing the emptying of the stomach, and reducing stomach acid.
Other parts of the ECS also have an effect in motility. The enzyme fatty acid amide hydrolase (FAAH) normally breaks down the endogenous cannabinoid called anandamide – this is the cannabinoid humans make that binds strongly to CB1. Several known phytocannabinoids can block FAAH and allow anandamide to have a longer action. This can enhance some of the positive benefits of CB1 activation in the gut in an indirect manner. If there is active inflammation in the gut, the body will also recruit CB2 receptors to help regulate motility that might be out of balance due to inflammation.(6, 7)
Conversely, there is evidence that substances that antagonize CB1 increase GI motility. This might make them useful agents in conditions such as gastroparesis or irritable bowel with constipation (IBS-C) where hypomotility is a dominant aspect of the pathology.(8)
Researchers have also proposed that there may be other, yet-to-be-identified mechanisms by which the ECS regulates motility. For example, the compound palmitoylethanolamide (PEA), a cannabinoid-like substance that is not known to interact with CB1 or CB2, seems to have similar effects on GI motility to THC. Animal studies have shown that it can slow motility in both the presence and absence of inflammation.(9) Because PEA is non-euphoria producing, this may make it a future alternative THC for those who which to avoid its use.
Regulates Communication with the Brain
You’ve no doubt become very familiar with the “gut-brain connection.” Many researchers now believe that connection is the ECS.(10) The enteric nervous system – sometimes called The Brain in the Gut – contains all the components of the ECS including CB1 and CB2 receptors, synthesizing and degrading enzymes, and endocannabinoids. The ECS regulates communication between the gut and the brain in a bi-directional manner.(8) This means that changes in the brain due to things like stress or pain can alter GI function and may be important in functional bowel health and health concerns like irritable bowel. Conversely, changes in the gut due to inflammation or infection are communicated back to the brain via the ECS. Deficiency of the ECS may be one reason that some chronic GI challenges can be hard to resolve.
The ECS seems to underpin the relationship between stress and visceral pain, inflammation and alterations in motility. Chronic stress induces wide-spread changes to the ECS that include a decrease in AEA, an increase in 2-AG and reduction in the number of CB1 receptors. This corresponds with changes in the hypothalamic-pituitary-adrenal (HPA) axis. The result is a decrease in endocannabinoid-mediated analgesia and a lessened ability to dampen visceral pain signals.(11) Decreased activity and number of CB1 receptors are likewise linked to increased intestinal motility, diarrhea, and nausea. This stress-induced ECS-HPA dysfunction, is thought to at least partially underlie the pathophysiology of irritable bowel syndrome.
Weight, Energy Balance and Metabolism
In the late 1990s and early 2000s as the science of the ECS was being revealed, one strong early area of interest was energy balance and metabolism. It was already known that cannabinoids in marijuana acted to stimulate appetite, a very useful function for individuals who have a medical condition that causes reduced appetite and unwanted weight loss. It was ultimately discovered that CB1 receptors signal activity that regulates energy homeostasis, particularly the release of Ghrelin and also of Neuropeptide Y – both potent stimulators of appetite and hunger.
But it was not long before drug researchers began asking, “What if we could create a drug that bound to the CB1 receptor and had the opposite effect? Wouldn’t that be great for weight loss?” So, they set out to make an “anti-munchies” drug – and rimonabant was born – an “inverse agonist” that created an opposing effect at the CB1 receptor and squelched appetite. Rimonabant worked – many individuals in the trials had significant weight loss and reversal of metabolic and cardiometabolic disease. However, dropout rates were high – more than 50 percent. In addition to suppressing appetite and benefitting metabolism, about 10 percent of those who took it also became severely depressed – even suicidal. They also developed pain, anxiety, irritability, nausea, and vomiting.(12) This is because in creating an opposite action at the CB1 receptor, rimonabant didn’t just suppress appetite – it suppressed all of the activities of this part of the ECS. The CB1 receptor is deeply involved in hedonics, thus when CB1 receptors were totally blocked, many people lost the joy that comes from eating – sometimes so much so that it was fatal. CB1 also plays a role in the regulation of pain, inflammation, and digestive function – which is why blockade of the receptor produced pain, nausea, and other symptoms of an ECS thrown completely out of balance.
While rimonabant was withdrawn from the market only a couple of years after it arrived, the interest in manipulating the ECS to alter appetite and metabolic function has not gone away. Obesity – especially central obesity – is generally characterized by a hyperactive, deranged ECS. In addition to its substantial role in appetite, CB1 stimulation increases fatty acid synthesis and hepatic lipogenesis, while reducing lipolysis. It also decreases insulin sensitivity, both in the liver and peripherally, mediated by a down-regulation of adiponectin.(13) Finally, it triggers increased production and storage of triglyceride. Couple all these actions with CB1 stimulation leading to amplified desire for indulgent foods and you have the makings of a vicious cycle that we often see in metabolic syndrome with obesity as the disease progresses.
While rimonabant was not the solution, it certainly pointed to the critical role of the ECS in weight and metabolic health. Several pharmacological agents are in various stages of clinical investigation and more will likely come. As for natural compounds, Tetrahydrocannabivarin (THCV) from cannabis is notable in its ability to act as a negative modulator of the CB1 receptor. In animal models it has been shown to improve insulin sensitivity and obesity and also act to protect pancreatic islet cells.(14) Cannabidiol (CBD) is also of interest based on the evidence that it antagonizes the action of CB1 by setting up a pharmacologic blockage that effectively dampens the overall impact of THC.(15)
Intestinal Permeability
The intestinal barrier represents a complex interface between self and non-self. As such the integrity of this barrier and how it is regulated is important to broad aspects of health from local gut health to metabolism and mental function. CB1 and CB2 are both expressed in the intestinal barrier and may play separate but related roles in its function. CB1 is thought to play a protective role at least in part through regulation of intestinal secretory IgA. In animal models where CB1 is blocked, IgA decreases, proinflammatory enzyme activity increases, and there is observable increase in intestinal permeability. Similar findings can be seen with an acute stress exposure. (15) In response to inflammation, CB1 expression is enhanced as is FAAH activity. This may be an effort by the body to slow motility, but this response also appears to be protective in other ways.
CB2 receptors also play a role, but primarily after an insult has occurred to the intestinal barrier. The expression of CB2 receptors has been shown to increase in conditions such as Crohn’s disease and ulcerative colitis where inflammation is a hallmark and intestinal permeability is disturbed. This is likely an effort by the body to bring inflammation under control due to the role of the CB2 receptor in decreasing the secretion of inflammatory cytokines (16). Animal models also suggest that an increased expression of CB2 receptors may reduce visceral pain sensation, suggesting a possible role for substances that interact with the CB2 receptor in managing both pain and inflammation in these conditions. Beta-caryophyllene (previously mentioned under inflammation) has already shown promise in this area.(17) Also notable is that some probiotics such as Lactobacillus acidophillus NCFM are able to activate the CB2 receptor which may account for some of the efficacy of probiotics in treating conditions with increased intestinal permeability and inflammation.
Perhaps some of the most exciting research on the ECS examines the regulation of the intestinal barrier and the interrelationship with the microbiome. In addition to the afore-mentioned interaction of some probiotics with ECS receptors, the ECS has now attracted attention as the missing link tying together the microbiome, intestinal barrier health, and systemic disease. For example, Akkermansia muciniphila is a bacterium that is associated with being protective against obesity, metabolic syndrome, and diabetes. In animal models, treatment with A.muciniphila has been shown decrease inflammatory markers, and metabolic endotoxemia by improving the integrity of the intestinal mucosal barrier.(18) So we know these systems are talking to each other, but not how.
The ECS may provide the answer. What is now proposed is that increases in intestinal permeability brought about obesity induced changes to the microbiome (such as decreases in A.muciniphila) trigger an upregulation in CB1 activity – particularly in adipose tissue – that further drives obesity and metabolic derangement. This is thought to be facilitated by the rise in serum lipopolysaccharide (LPS) which directly stimulates ECS activity. Experimental models indicate that addressing leaky gut with pre or probiotics may offer a valid intervention if intestinal permeability can be reversed resulting in a fall in LPS and normalization of ECS balance. (19)
In conclusion, the endocannabinoid system exerts strong influences on digestive health. As we further understand the mechanisms of the ECS in the gut, we can leverage therapeutic interventions that directly target this influential system, allowing us to address a number of conditions that, while common, are often challenging to address in clinical practice.
 Doctor Jacqueline Jacques is a Naturopathic Doctor (NCNM 1997) with over 20 years of expertise in medical nutrition. She has spent much of her career in the supplement industry as a formulator, speaker, writer, and educator. Prior to joining Thorne Research where she is the SVP of Portfolio Development, she was Chief Science Officer for Bariatric Advantage, a company dedicated to providing nutritional care to weight loss surgery patients. Doctor Jacques has appeared as a guest on radio and television, and has spoken nationally and internationally to health professionals and the public alike. She is the author of the book Micronutrition for the Weight Loss Surgery Patient, and has contributed to numerous other professional publications. Additionally, she has served on the boards/advisory boards for the Obesity Action Coalition, the Samueli Center for Integrative Medicine, YOR Health, Bodywise International, and the California Naturopathic Doctor’s Association (CNDA). She resides in Southern California with her two teenaged boys.
Doctor Jacqueline Jacques is a Naturopathic Doctor (NCNM 1997) with over 20 years of expertise in medical nutrition. She has spent much of her career in the supplement industry as a formulator, speaker, writer, and educator. Prior to joining Thorne Research where she is the SVP of Portfolio Development, she was Chief Science Officer for Bariatric Advantage, a company dedicated to providing nutritional care to weight loss surgery patients. Doctor Jacques has appeared as a guest on radio and television, and has spoken nationally and internationally to health professionals and the public alike. She is the author of the book Micronutrition for the Weight Loss Surgery Patient, and has contributed to numerous other professional publications. Additionally, she has served on the boards/advisory boards for the Obesity Action Coalition, the Samueli Center for Integrative Medicine, YOR Health, Bodywise International, and the California Naturopathic Doctor’s Association (CNDA). She resides in Southern California with her two teenaged boys.
References:
-
Russo E. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions?. Neuro endocrinology letters. 2008 Apr;29(2):192-200
-
Hasenoehrl C, Storr M, Schicho R. Cannabinoids for treating inflammatory bowel diseases: where are we and where do we go? Expert review of gastroenterology & hepatology. 2017 Apr 3;11(4):329-37.
-
Di Marzo, V., & Izzo, A. A. (2006). Endocannabinoid overactivity and intestinal inflammation. Gut, 55(10), 1373-6.
-
Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nature Reviews Immunology. 2005 May;5(5):400.
-
Borrelli F, Fasolino I, Romano B, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochemical pharmacology. 2013 May 1;85(9):1306-16.
-
Izzo A, Sharkey K. Cannabinoids and the gut: new developments and emerging concepts. Pharmacology & therapeutics. 2010 Apr 1;126(1):21-38.
-
Sharkey K, Wiley J. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology. 2016 Aug 1;151(2):252-66.
-
Pertwee R. Cannabinoids and the gastrointestinal tract. 2001; 48:859–867.
-
Capasso R, Izzo A, Fezza F, et al. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. British journal of pharmacology. 2001 Nov 1;134(5):945-50.
-
Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature medicine. 2007 Jan;13(1):35.
-
Sharkey K, Wiley J. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology. 2016 Aug 1;151(2):252-66.
-
Gadde K, Allison D. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation. 2006 Aug 29;114(9):974-84.
-
Gruden G, Barutta F, Kunos G, Pacher P. Role of the endocannabinoid system in diabetes and diabetic complications. British journal of pharmacology. 2016 Apr;173(7):1116-27.
-
Wargent E, Zaibi M, Silvestri C, et al. The cannabinoid Δ 9-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutrition & diabetes. 2013 May;3(5):e68.
-
Zoppi S, Madrigal J, Pérez-Nievas B, et al. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011 Dec 1;302(5):G565-71.
-
Wright K, Duncan M, Sharkey K. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2007;153(2):263-70.
-
Bento A, Marcon R, Dutra R, et al. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. The American journal of pathology. 2011 Mar 1;178(3):1153-66.
-
Cani P, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Current opinion in pharmacology. 2013 Dec 1;13(6):935-40.
-
Cani P. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clinical Microbiology and Infection. 2012 Jul;18:50-3.





