The Annals of Internal Medicine reports that accurate serologic tests to detect host antibodies to severe acute respiratory syndrome–related coronavirus-2 (SARS-CoV-2) are critical for the public health response to the disease pandemic.

Despite the number and availability of serologic assays that test for antibodies against SARS-CoV-2, most have only undergone minimal external validation to date. “This hinders assay selection and implementation, as well as interpretation of study results,” they write.
“In addition, critical knowledge gaps remain regarding serologic correlates of protection from infection or disease, and the degree to which these assays cross-react with antibodies against related coronaviruses. This article discusses key use cases for SARS-CoV-2 antibody detection tests and their application to serologic studies, reviews currently available assays, highlights key areas of ongoing research, and proposes potential strategies for test implementation.”
Summary of what you will learn from this study:
- Molecular testing on respiratory specimens is hampered by imperfect sensitivity and limited testing capacity.
- Antibody testing should not be used as the sole basis for diagnosis of acute COVID-19.
- Validation of novel antibody detection tests for SARS-CoV-2 must pay careful attention to the choice of source populations and reference standards, and to possible cross-reactivity with antibodies to other human coronavirus infections.
- Plaque reduction neutralization assays are currently the reference standard for determination of host antibodies capable of inhibiting viral replication, but must be performed in a biosafety level 3 laboratory.
- Urgent research is needed to determine the serologic correlates of immunity against SARS-CoV-2.
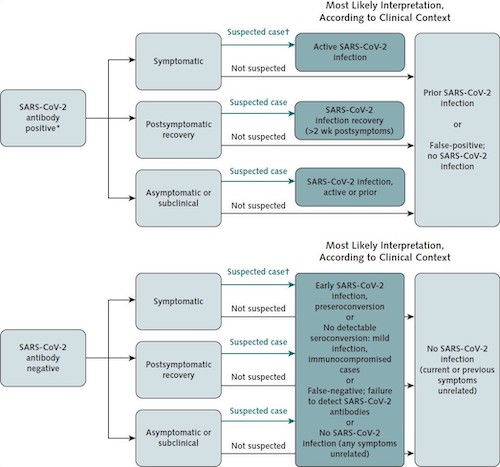
* The relationship between positive antibody results and protective immunity will vary among assays and must be validated individually.
† Includes high exposure, high risk, hot spots, and contact tracing.
Conclusion/ “In conclusion, the COVID-19 pandemic has revealed several gaps in our diagnostic arsenal and is highlighting the essential role of serodiagnostics as part of our public health response. With the use of carefully validated assays, appropriately designed serologic studies will help characterize transmission dynamics and refine disease burden estimates. Urgent scientific research is needed to link specific serologic variables with immunity against SARS-CoV-2.”
Click Here for Full Text Study