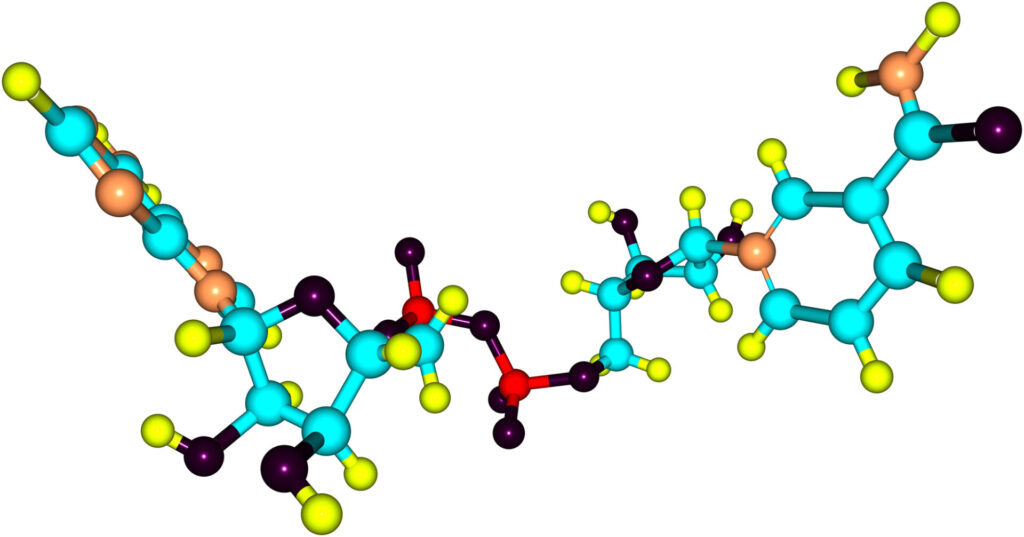
Nicotinamide adenine dinucleotide (NAD) is a key cellular component in virtually all forms of life. While best known for its major role in energy metabolism, NAD is also essential in many signaling pathways. As a result, many NAD-dependent proteins involved in signaling reactions serve as metabolic sensors in the cell and are involved in regulating downstream metabolic pathways.
Proteins involved in NAD-dependent signaling reactions, such as PARP1 which is essential for DNA repair, requires the cleavage of NAD. A multitude of studies have shown that NAD levels decline during aging, potentially resulting in metabolic dysfunctions and age-related diseases. A team of scientists from the University of Bergen has now investigated how cells respond to chronically depleted NAD levels, as observed during aging.
The study:
The research team, led by Professor Mathias Ziegler, developed models allowing them to study the effect of reduced cellular NAD levels. The study clearly demonstrates how NAD-pools within subcellular compartments are not isolated but rather interconnected through specific transport mechanisms and regulatory enzymes, which facilitate the intracellular transfer of NAD.
Moreover, the study emphasizes the role of mitochondria, best known as the “powerhouses of the cell,” as regulators of the NAD-pools, helping to maintain cellular NAD homeostasis. Mitochondria can act as reservoirs, ensuring that NAD levels remain stable in other cellular compartments. This allows cells to adapt to fluctuations in NAD demand, such as under conditions of metabolic stress. This function is vital for securing NAD availability as needed for various cellular processes, including cellular energy transfer and signaling to control gene expression and DNA repair.
These findings further extend the understanding of cellular metabolism and health. Stable levels of cellular NAD across subcellular compartments are a key factor for cellular health. Whereas mitochondria, losing the capability to use the mitochondrial NAD reservoir for maintaining the balance and distribution of NAD across subcellular compartments due to mitochondrial dysfunction can be detrimental to cells. Moreover, decreased NAD levels can cause metabolic dysfunctions, contributing to aging and the development of diseases such as neurodegenerative disorders and even cancer. As the balance of cellular NAD seems to be regulated by NAD-dependent enzymes rather than other regulatory mechanisms, this enables novel therapeutic approaches for maintaining NAD levels to promote cellular health and longevity.
Conclusions:
Based on their new findings, the team of researchers believes that excessive consumption of mitochondrial NAD might constitute a key factor leading to dysfunctional mitochondria and thus aging-associated diseases.
Indeed, initial clinical trials in Norway and internationally using therapeutic supplementation approaches aiming to increase NAD levels have provided encouraging results.
“We are very excited about having discovered yet another mechanism potentially involved in disease development and progression,” says the first author, Høyland.
Professor Zielger added, “Our study also demonstrates the importance of basic research to identify promising targets to slow ageing and to treat ageing-related diseases.”
The results have been published in the renowned Journal Nature Metabolism and featured in a News and Views article in the same issue.