The Evidence Behind Natural Interventions for Non-alcoholic Fatty Liver Disease: Botanicals, Nutrients, and Probiotics for Treating This Common Ailment
Given the rise in non-alcoholic fatty liver disease (NAFLD) in both adults and pediatrics and the lack of an indicated pharmaceutical treatment,[1],[2] natural strategies for the treatment of this condition stand well-poised as the “next best thing.” Several nutritional supplements and botanicals have been studied clinically and show promise for treatment of NAFLD and its inflammatory counterpart, non-alcoholic steatohepatitis (NASH).[3] Given how many of these therapies address the factors that contribute to the development of NAFLD, it doesn’t take much reasoning to understand how they may effectively address some of the root causes of disease, rather than just addressing a symptom.
Five supplemental interventions with clinical or epidemiological evidence for their use in the setting of NAFLD are phosphatidyl choline, which provides phospholipids and choline, vitamin E, both as alpha tocopherols and a blend of delta and gamma tocotrienols, milk thistle seed extract, berberine, and probiotics.
Fatty Liver Changes: Correlated with a Phosphatidyl Choline Deficiency?
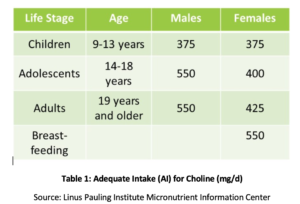
Phosphatidyl choline (PC) is essential to the health of all the cells in the body and is one of the main phospholipids of which cellular membranes are comprised.[4],[5] PC comprises over 90% of the total bile phospholipids content and facilitates fat emulsification, absorption, and transport.[6] Studies have shown that the recommended adequate intake (AI) of choline (see Table 1) may not be sufficient for prevention of symptoms of deficiency such as fatty liver changes or muscle damage.[7] Additionally, a recent study found that only 8% of US adults meet the recommended AI of choline, with vegetarians, postmenopausal women, and men at greatest risk of inadequacy.[8],[9] Genetic polymorphisms also may increase the need for choline,[10] which also is a source of methyl groups in the body.
In postmenopausal women with NAFLD, decreased choline intake has been shown to be significantly associated with an increased risk of fibrosis.[11] In animals, PC has been shown to protect against fibrosis associated with hepatic inflammation.[12],[13] Increased intake of PC has been shown to enhance biliary lipid secretion, thereby preventing cholestasis and subsequent liver damage via numerous mechanisms, including suppression of nuclear factor kappa B (NF-κB), a well understood molecular trigger of inflammation.[14],[15] In an animal model of biliary obstruction, supplemental PC has been shown to reduce liver damage, ductal proliferation, and fibrosis.[16]
PC is also essential for the health of the gut and is a primary component of the protective intestinal mucus layer.[17] In cell cultures, treatment with PC has been shown to reduce the migration of endotoxin through intestinal epithelial cells, further suppressing the associated inflammatory cytokine response.[18] PC also reduces endotoxin translocation in the setting of alcohol-induced increases in intestinal permeability.[19] This beneficial effect has been proposed as one of the mechanisms by which PC may be considered therapeutic in alcoholic liver disease.
Vitamin E
As an oxidative stress hit and diminished antioxidant defences has been proposed as a factor leading the development of NAFLD,[20] it should not be surprising that antioxidants have been investigated as a potential treatment for the condition.
 Vitamin E has been studied in several clinical trials for the treatment of NAFLD, both as a standalone and adjunctive therapy. In a review of these studies, the dosage of vitamin E ranged from 400 to 1200 IU/day and study duration was from 24 weeks to more than two years.[21] Findings generally were positive, showing that when vitamin E was included, improvements in histology, steatosis, and/or transaminase levels were seen.[22],[23] In pediatrics with NAFLD, vitamin E has also been studied as a therapy. In an open-label pilot study including 11 children with NAFLD, supplementation of between 400 to 1200 IU of vitamin E for four to ten months was found to normalize transaminase and alkaline phosphatase levels during treatment, however they returned to abnormal once treatment was stopped.[24] Hepatic echogenicity did not change during the course of treatment.
Vitamin E has been studied in several clinical trials for the treatment of NAFLD, both as a standalone and adjunctive therapy. In a review of these studies, the dosage of vitamin E ranged from 400 to 1200 IU/day and study duration was from 24 weeks to more than two years.[21] Findings generally were positive, showing that when vitamin E was included, improvements in histology, steatosis, and/or transaminase levels were seen.[22],[23] In pediatrics with NAFLD, vitamin E has also been studied as a therapy. In an open-label pilot study including 11 children with NAFLD, supplementation of between 400 to 1200 IU of vitamin E for four to ten months was found to normalize transaminase and alkaline phosphatase levels during treatment, however they returned to abnormal once treatment was stopped.[24] Hepatic echogenicity did not change during the course of treatment.
Tocotrienols are the lesser-studied family of the naturally-occurring forms of vitamin E, with the tocopherols, more often found in nature, dominating scientific research.[25] However, tocotrienols are potent antioxidants, quoted in some research as having 40 to 60 times the antioxidant potential of tocopherols. Tocotrienols have been shown to improve total cholesterol and its fractions, reducing total and LDL cholesterol by 15 to 20%,[26],[27] and triglycerides up to almost 30%.[28] Tocotrienols have also been shown to have anti-inflammatory and antioxidant effects in vivo, lowering high-sensitivity C-reactive protein (hs-CRP) levels and reducing LDL oxidation.
Tocotrienols have also been studied as a treatment for NAFLD. In patients with ultrasound-diagnosed NAFLD, 200 mg of mixed tocotrienols (sourced from palm oil, with a high gamma fraction and an additional 61 mg of alpha tocopherols) taken twice daily for one year was shown to significantly normalize hepatic echogenic response and rate of remission compared to placebo.[29] In a second study, including patients with ultrasound-diagnosed NAFLD and transaminase elevation, 300 mg of tocotrienols (a 90:10 delta:gamma blend) taken twice daily for 12 weeks significantly decreased aspartate aminotransferase (AST), alanine aminotransferase (ALT), hs-CRP, and malondialdehyde (a marker of oxidative stress) levels as well as fatty liver index score compared to placebo.[30]
Milk Thistle
Milk thistle (Silybum marianum) is possibly the most well-known liver-protective botanical. Silymarin, a mixture of the active constituents of milk thistle, and silibinin, the most active compound found within it, have been shown in animal studies to reduce liver injury caused by acetaminophen, alcohol, iron overload, and radiation among other known liver-toxic substances.[31] Silymarin has been shown to increase levels of glutathione, a powerful antioxidant, in both the liver and intestines.[32] It also reduces lipid peroxidation, which damages cellular membranes, altering their function, and possibly causing cellular death.[33]
Silibinin and silymarin have been shown to activate a nuclear bile acid receptor known as farnesoid X receptor (FXR) in the liver, down-regulating inflammatory pathways and correcting insulin resistance and dyslipidemia induced by high-fat diet (HFD) feeding.[34] FXR is a key regulator of bile acid, glucose, and lipid balance in the body.[35] Medications which interact with FXR in a similar manner to these milk thistle derived compounds are also being investigated for the treatment of NAFLD and the related metabolic challenges.[36]
Clinical studies have also shown milk thistle improves various parameters associated with NAFLD. A 2017 meta-analysis found that treatment with milk thistle significantly reduces ALT and AST by −5.08 IU/L and −5.44 IU/L, respectively, in patients with NAFLD.[37] Dosages ranged from 140 mg once a day to 200 mg three times a day, for a duration of 8 to 24 weeks. At the lowest dosage of 140 mg daily, after 8 weeks, significant improvements were seen in fasting blood glucose (FBG), lipid profile, and serum insulin levels in addition to AST and ALT reductions from 56 to 37.77 IU/L and 78.73 to 53.05 IU/L, respectively.[38]
Berberine
Berberine, the orangish-yellow alkaloid found in botanicals such as Oregon grape root and bark, goldenseal, and barberry, is another botanically-derived substance that has numerous mechanisms by which it may help protect against NAFLD and support its resolution.[39],[40],[41]
The antidiabetic and lipid-balancing effects of berberine have been demonstrated in several clinical trials,[42] and  may be means via which berberine positively affects liver function. Berberine has been shown to alter metabolism-related gene expression and bile acid metabolism via pathways involving FXR as well.[43] In animal studies, berberine has been shown to have the effect of preventing HFD-associated obesity and hepatic triglyceride accumulation in wild-type (normal) mice, but not in those that had the genetic elimination of intestinal FXR expression. Berberine has also been shown to suppress obesity-associated inflammation and hepatic steatosis in mice by decreasing phosphorylation of the inflammatory complex known as JNK1,[44] a protein kinase implicated in the development of steatohepatitis.[45] JNK1 is strongly activated by environmental stressors and pro-inflammatory cytokines.
may be means via which berberine positively affects liver function. Berberine has been shown to alter metabolism-related gene expression and bile acid metabolism via pathways involving FXR as well.[43] In animal studies, berberine has been shown to have the effect of preventing HFD-associated obesity and hepatic triglyceride accumulation in wild-type (normal) mice, but not in those that had the genetic elimination of intestinal FXR expression. Berberine has also been shown to suppress obesity-associated inflammation and hepatic steatosis in mice by decreasing phosphorylation of the inflammatory complex known as JNK1,[44] a protein kinase implicated in the development of steatohepatitis.[45] JNK1 is strongly activated by environmental stressors and pro-inflammatory cytokines.
Berberine also acts in the gut. It affects not only the gut microbial balance,[46] but also directly influences intestinal permeability, improving tight junction integrity in animals subject to endotoxemia or cell cultures treated with pro-inflammatory cytokines.[47],[48]Direct anti-inflammatory effects have also been demonstrated.[49]
The benefits of berberine in NAFLD have also been demonstrated clinically in a randomized, parallel controlled, open-label clinical trial.[50] In patients with NAFLD, berberine was shown to restore normal hepatic architecture, lipid, and blood sugar metabolism, with significant improvements seen over the population who only implemented lifestyle changes.
Probiotics
Given the relationship between the many digestive system disturbances and liver enzyme elevation, it is not surprising that probiotics also have been studied as a treatment for NAFLD. A recent meta-analysis well summarizes the collective findings.[51]
One-hundred thirty-four patients diagnosed with NAFL/NASH by liver biopsy were included in this analysis, and each intervention used in the four randomized, controlled trials eligible for this meta-analysis was unique (Lactobacillus bulgaricus and Streptococcus thermophilus for three months; Lactobacillus GG for eight weeks; Bifidobacterium longum and fructooligosaccharides (FOS) for 24 weeks; and a proprietary combination of Lactobacillus plantarum, L. delbrueckii, L. acidophilus, L. rhamnosus, and Bifidobacterium bifidum for six months). The dosage of probiotics ranged from 500 million to 12 billion colony-forming units (CFUs) daily. The probiotic treatments were shown to significantly decrease ALT and AST levels by –23.71 UI/L and –19.77 UI/L, respectively. Significant improvements in total cholesterol, tumor necrosis factor (TNF)-α levels, and insulin resistance were also noted.
Two additional clinical trials investigated probiotics as a treatment for pediatric NAFLD. In a double-blind, placebo-controlled, pilot study, twelve children, having an average age of 10.7 years old, with ultrasound-diagnosed fatty liver changes and persistent transaminase elevation were given 12 billion CFUs of L. rhamnosus GG or placebo daily for 8 weeks. Treatment with the probiotic significantly reduced ALT levels compared to placebo, however liver echogenicity and AST levels did not change. Additionally, anti-peptidoglycan-polysaccharide antibodies, an indicator of bacteria or bacterial membrane translocation through the intestinal barrier, significantly decreased in children receiving the probiotic compared to placebo.[52] The second study investigated treatment of children having biopsy-proven NAFLD with VSL #3, a high potency blend of 8 probiotic strains (including Streptococcus thermophilus, 3 Bifidobacteria spp., and 4 Lactobacillus spp.), compared to placebo.[53] In children receiving the probiotic, fatty liver scores were significantly improved with the probability of none, light, moderate, or severe fatty liver at the end of the study being 21%, 70%, 9% and 0% compared to 0%, 7%, 76% and 17% in the placebo group.
Conclusion
There is a wide range of safe and effective options available that support the restoration of health in those with NAFLD. Although each individual may have different underlying mechanisms contributing to hepatic dysfunction and inflammatory changes, each of these supportive natural agents—and many others (see Table 2)—may support normal liver function in individuals with these challenges.
Table 2: Nutritional and botanical interventions for NAFLD. Nutrients such as these should be used under the guidance of a qualified and licensed healthcare practitioner.
| Nutrient | Dosage | Mechanism |
| Phosphatidylcholine (PC), a source of dietary choline | 1.5 g twice daily with meals (PC) OR 200 mg twice daily (choline) | Choline is a common dietary deficiency. PC is necessary for production of bile and protective gastrointestinal mucosa barrier. |
| Milk thistle seed | 140 to 200 mg once to three times daily | Hepatoprotective. Supports hepatic glutathione levels, stabilizes bile salt export pump (BSEP), and activates FXR pathways. |
| Berberine HCl | 500 mg two to three times daily[54] | Improves serum glucose and lipid profiles, also reducing hepatic fat content. |
| Probiotics, including strains such as B. longum, B. bifidum, S. thermophilus, L. rhamnosus, L. acidophilus, and L. plantarum | 12 billion colony-forming units (CFUs) daily | Improved gut epithelial barrier function and reduced intestinal and systemic inflammation.[55] |
| Acetyl-glutathione | 300 mg daily[56] | Improves detoxifying ability of hepatocytes.[57] |
| N-acetylcysteine (NAC) | 500–600 mg twice daily, best taken on an empty stomach | NAC blocks the propagation of lipid peroxidation and supports hepatic glutathione levels.[58] |
| Vitamin E (tocopherols)
OR Tocotrienols |
400 to 1200 IU daily with food
200 to 300 mg twice daily with food |
Protective antioxidant.
Tocotrienols attenuate triglyceride accumulation by regulating fatty acid synthase and carnitine palmitoyltransferase enzymes, leading to a reduction of hepatic inflammation and endoplasmic reticulum stress.[59] |
| Omega-3 essential fatty acids | 2 – 4 g daily, with meals
|
Omega-3 polyunsaturated fatty acids are known to downregulate sterol regulatory element-binding protein-1c and upregulate peroxisome proliferator-activated receptor alpha, thus favoring fatty acid oxidation and reducing steatosis.[60] |