From the plaque on our teeth to the mucosal layers in the gut lumen, biofilms are everywhere in our environment — and they are a major threat to our health. According to the National Institutes of Health, more than 65% of all microbial and chronic infections are associated with biofilm formations.
But what exactly are biofilms? In simple terms, biofilms are a group of organisms, including bacteria, viruses, fungi, and parasites, that form a community around damaged, infected areas of the body. Common health issues that involve biofilms include:
- Kidney stone development
- Otitis Media infections
- Endocarditis
- Diabetic ulcerations
… and many more
Biofilms adhere to a substrate or matrix that is not necessarily alive or even organic. They are notorious for forming on implanted medical devices made from inert plastics, such as indwelling catheters. Biofilm infections affect millions of people, primarily in the developed world. These include pneumonia in cystic fibrosis patients; chronic wounds; chronic otitis media, prostatitis, or sinusitis; and implant- and catheter-associated infections. Since biofilms are so resistant to treatment, understanding their biology is vital.
Biofilms: “Best Described as Slime”
Here is a great explanation of biofilms from a paper published in the Natural Medicine Journal by Jacob Schor, ND, FABNO, and Lise Alschuler, ND, FABNO:
“Certain microorganisms, in particular certain species of bacteria and yeast, will convert or coalesce from individual free-living single individuals into an aggregate of cells. These aggregates are bound together by a film of secreted material — ‘a hydrated polymeric matrix of their own synthesis to form biofilms’ — best described as slime. Thus, bacteria (and some yeast) can exist in 2 different life forms. In 1 form, the individual bacteria exist as single, independent, or planktonic cells and in the second form, the bacteria organize into sessile aggregates.”
In an article titled “Bacterial Biofilms: A Common Cause of Persistent Infections,” JW Costerton at the Center for Biofilm Engineering in Montana defines a bacterial biofilm as “a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface.”
In lay terms, that means bacteria can join on any surface and start to form a protective matrix around the group. The matrix is made of polymers — substances composed of molecules with repeating structural units that are connected by chemical bonds. So distinct species of bacteria, yeast, and viruses can work together to form communities or colonies that enable individual organisms to survive and thrive more effectively than if they were living on their own.
In these biofilm communities, anaerobic bacteria can attach to inert surfaces, but they then facilitate the development of an environment in which aerobic bacteria can form. And it gets even more complicated, as these diverse organisms can then start exchanging genetic material.
These biofilms can develop into chronic, infectious conditions — many of which we deal with clinically, such as endocarditis, diabetic ulceration, infections on the surfaces of implantable devices, etc.
“Acute infections are assumed to involve planktonic bacteria and are treatable with antibiotics. As sessile biofilms, the same bacterial infections often develop into a chronic state, untreatable with antibiotics and capable of evading the body’s defenses,” said Jacob Schor, ND, FABNO, and Lise Alschuler, ND, FABNO, in their article from the Natural Medicine Journal.
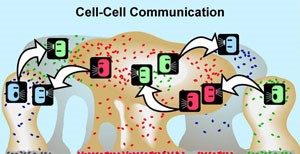
The Phenomenon of Quorum Sensing
According to a review in the journal Discoveries, multiple studies have shown that during the time a biofilm is being created, the pathogens inside it can communicate with each other thanks to a phenomenon called quorum sensing.
Although the mechanisms behind quorum sensing are not fully understood, the phenomenon allows a single-celled bacterium to perceive how many other bacteria are in close proximity. If a bacterium can sense that it is surrounded by a dense population of other pathogens, it is more inclined to join them and contribute to the formation of a biofilm.
Bacteria that engage in quorum sensing communicate their presence by emitting chemical messages that fellow infectious agents can recognize. When the messages grow strong enough, the bacteria respond En masse, behaving as a group.
Quorum sensing can occur within a single bacterial species or between diverse species and can regulate a host of different processes, serving as a simple communication network. A variety of different molecules can be used as signals.
Biofilm “Persisters”
When bacteria become involved in a biofilm, their usual behavior changes. A prime example of this is that the bacteria rarely divide/reproduce — this is part of the reason biofilms are so resistant to standard antibiotic therapies.
In fact, depending on the organism and type of antimicrobial and experimental system, biofilm bacteria can be up to a thousand times more resistant to antimicrobial stress than free-swimming bacteria of the same species.
Even if a course of heavy antibiotics is successful, several cells called “persisters” are left behind that remain alive and viable and can then start the process of division and repopulation. This is yet another survival mechanism that biofilms employ.
“Though biofilms are essential for the growth and survival of healthy flora, unfriendly bugs can also use these structures to repopulate and are the root of many long-term, recurrent, and reluctant infections. If you hear or utter the phrase, ’I get better for a while and then all my symptoms come back,’ think biofilm issues. No number of antimicrobial agents, pharmaceutical or herbal, properly address these issues. You must break down the structure of these biofilms so the antimicrobial agents can do their best work,” says Dr. Leah Linder with SFI Health.
The Biofilm Lifecycle
If you look at the typical lifecycle of a biofilm, it is easier to understand how patients with cardiovascular and other conditions experience radical transformations in their health after having a root canal — because the biofilms can be removed from the body once the root canal material (substrate) is removed.

Nodes of relapsing infection? Researchers often note that, once biofilms are established, planktonic bacteria may periodically leave the biofilm on their own. When they do, they can rapidly multiply and disperse.
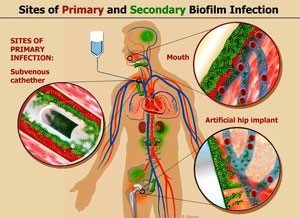
Biofilms & Lyme Disease
Lyme spirochetes tend to hide out in biofilms, remaining dormant. This helps explain why antibiotics can be ineffective once the biofilm starts to develop (and why it is important to treat Lyme infections aggressively early on).
As mentioned above, biofilms are also a significant issue for implanted medical devices such as prosthetic joints, mechanical heart valves, IUDs, catheters, etc.
Therapeutic Solutions for Biofilms
Pharmaceutical research mostly centers on developing compounds that act as quorum-sensing disruptors, as well as exploring pulsed low-dose antibiotic protocols. Fortunately, there are natural treatments that can help break up and eradicate biofilms. A big benefit of these holistic therapies is that they do not create the well-known microbiome imbalances typical with long-term antibiotic usage.
“In some ways, biofilms are one of your body’s short-term survival mechanisms: Isolating a problem area so that it does not spread. But, in a great paradox, biofilms also create microenvironments where cancer, infections, toxins, and inflammation can hide — untouched by your body’s defense and repair mechanisms,” says Dr. Isaac Eliaz, author of The Survival Paradox. “When you disrupt and break down biofilms, you can allow your immune system, medicines, and integrative therapies to do what they do best — repair, rejuvenate, and heal you.”
Standout natural compounds for addressing biofilms include:
- Berberine
- Mastic Gum
- Cloves
- Garlic
- Oregano oil (carvacrol being the key active ingredient)
- Artemisia (wormwood)
- Curcumin
- Reserpine
- Linoleic Acid
- Serrapeptase proteolytic enzymes
- α-amylase
- Lactoferrin ties up iron, away from bacteria
- Bismuth
- Chelation removes many heavy metals & weakens the structure of biofilms
- N-acetyl-L-cysteine (NAC)
- Flavonoids
- Resveratrol
- Cranberry proanthocyanidins
- Chlorogenic acid
- Boswellia
Phages: One novel approach for biofilms is the use of phages (or bacteriophages) — viruses that can denature certain types of bacteria and biofilms. Interestingly, phage therapy was developed decades ago in Europe, but the approach fell out of favor with the advent of antibiotics.
Metabolomics: This emerging field helps provide valuable information on the composition of biofilms and how to best target therapeutic interventions.
Biofilms are mysterious and complex — there is a lot that we still do not know, and undoubtedly, there will be more research on this topic in the future. There is no question that biofilms play a significant role in human health and the development of disease. So, it is crucial that we all learn as much as possible about biofilms to help patients experience maximum health and wellness.
Resources
- Monroe, D. Looking for Chinks in the Armor of Bacterial Biofilms. 2007 Nov. DOI: 10.1371/journal.pbio.0050307
- Nat Prod Rep. 2010 Mar;27(3):343-69. doi: 10.1039/b804469b. Epub 2010 Feb 3
- Dickschat JS,Quorum sensing and bacterial biofilms
- APMIS Suppl. The role of bacterial biofilms in chronic infections. 2013 May;(136):1-51. doi: 10.1111/apm.12099.
- Bjarnsholt, T. Bacterial biofilms: why should we care?
Sci Prog. Bacterial biofilms and human disease. 2001;84(Pt 3):235-54. - Wilson M Determining the potential use of biosurfactants in preventing endodontic infections
Bio
Robert Lamberton, a Master Formulator of professional-grade supplements, works with select companies, clinics, and practitioners to develop cutting-edge formulations. He is a certified Functional Nutritional Therapy Practitioner (FNTP) who specializes in helping patients resolve complex health issues, optimizing their quality of life, and reversing their biological age. He has been a guest instructor and lecturer at Boucher Naturopathic Medical School (BINM) in Vancouver, BC. Lamberton also produces a weekly newsletter for practitioners that covers the latest published research, including information that has yet to reach the clinical community. Learn more at RobLamberton.com.
Copyright © 2023 R. V. Lamberton & Associates, All rights reserved.