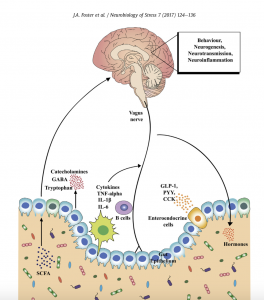
At the 2016 Neurobiology of Stress Workshop in Newport Beach, CA, a group of experts presented the symposium The Microbiome: Development, Stress, and Disease, published in Mammalian Genome. This report, coauthored by leading experts Jane Foster, PhD, John Crynan PhD and colleagues, summarizes and builds upon some of the key concepts in that symposium within the context of how microbiota might influence the neurobiology of stress.
The importance of the gut-brain axis in regulating stress-related responses has long been appreciated. More recently, the microbiota has emerged as a key player in the control of this axis, especially during conditions of stress provoked by real or perceived homeostatic challenge. Diet is one of the most important modifying factors of the microbiota-gut-brain axis. The routes of communication between the microbiota and brain are slowly being unravelled, and include the vagus nerve, gut hormone signaling, the immune system, tryptophan metabolism, and microbial metabolites such as short chain fatty acids.
The importance of the early life gut microbiota in shaping later health outcomes also is emerging. Results from preclinical studies indicate that alterations of the early microbial composition by way of antibiotic exposure, lack of breastfeeding, birth by Caesarean section, infection, stress exposure, and other environmental influences – coupled with the influence of host genetics can result in long-term modulation of stress-related physiology and behaviour.
The gut microbiota has been implicated in a variety of stress- related conditions including anxiety, depression and irritable bowel syndrome, although this is largely based on animal studies or correlative analysis in patient populations. Additional research in humans is sorely needed to reveal the relative impact and causal contribution of the microbiome to stress-related disorders. In this regard, the concept of psychobiotics is being developed and refined to encompass methods of targeting the microbiota in order to positively impact mental health outcomes.
Introduction
The concept of the gut influencing brain and behaviour, and vice-versa, has perhaps been best appreciated and studied as it relates to the cephalic (preparatory) phase of digestion, visceral pain and malaise, and the ability of emotional stress to disrupt digestive functions. Nonetheless, despite wide integration of the “gut-brain” concept into our everyday vernacular (e.g., gut feelings, gut-wrenching, gut instinct, gutted, gutsy, it takes guts, butterflies in one’s stomach), neuroscientists have only recently developed adequate tools with which to reveal the bidirectional links between gut physiology and brain function, and to determine how these links operate under normal and stressful conditions.
The Dozen Concepts You Will Learn When Reading this Paper:
- The linkage between gut functions on the one hand and emotional and cognitive processes on the
Click on the image for a larger version other is afforded through afferent and efferent neural projection pathways, bi-directional neuroendocrine signaling, immune activation and signaling from gut to brain, altered intestinal permeability, modulation of enteric sensory-motor reflexes, and entero endocrine signaling.
- Gut microbiota have emerged as a critical component potentially affecting all of these neuro-immuno-endocrine pathways. Disease-related animal models such as mild and chronic social defeat stress also lead to significant shifts in cecal and fecal microbiota composition; these changes are associated with alterations in microbiota-related metabolites and immune signaling pathways suggesting that these systems may be important in stress-related conditions including depression.
- Evidence for a crucial role for the microbiota in regulating stress-related changes in physiology, behaviour and brain function has emerged primarily from animal studies.
- A complex communication network exists between the gut and the CNS, which includes the enteric nervous system (ENS), sympathetic and parasympathetic branches of the autonomic nervous system (ANS), neuroendocrine signaling pathways, and neuro- immune systems.
- The gut is innervated by the ENS, a complex peripheral neural circuit embedded within the gut wall comprising sensory neurons, motor neurons, and interneurons. While the ENS is capable of independently regulating basic gastrointestinal (GI) functions (i.e., motility, mucous secretion, and blood flow), central control of gut functions is provided by vagal and, to a lesser extent, spinal motor inputs that serve to coordinate gut functions with the general homeostatic state of the organism.
- Chronic treatment with Lactobacillus rhamnosus (JB-1) led to region-dependent alterations in central GABA receptor expression, accompanied by reduced anxiety- and depression-like behaviour and attenuation of stress-induced corticosterone response; these effects required an intact vagus nerve.
- A multitude of biologically active peptides are present at numerous locations throughout the brain-gut axis, and have a broad array of functions that include not only gut motility and secretion, but also regulation of emotional affect and stress resilience. Bacterial by-products that come into contact with the gut epithelium are known to stimulate enteroendocrine cells (EECs) to produce several neuropeptides such as peptide YY, neuropeptide Y (NPY), cholecystokinin, glucagon-like peptide-1 and -2, and substance P.
- The immune system plays an important intermediary role in the dynamic equilibrium that exists between the brain and the gut. The HPA axis, ANS and ENS all directly interact with the immune system, and the gut itself is an important immune organ that provides a vital defensive barrier between externally-derived pathogens and the internal biological environment.
- One of the principal mechanisms proposed to underlie a gut-brain link in stress-related disorders is via disrupted gut barrier function commonly known as the “leaky gut” phenomenon, which is proposed to contribute to MDD.
- Stress, including early life stress, is a key risk factor for IBS, the most common functional gastrointestinal disorder. IBS is considered to reflect pathologically altered gut-brain axis homeostasis.
- Acute and chronic exposure to stress can alter both the quality and quantity of calories consumed, and stress-induced alterations in food intake and energy balance can interact with emotional state. In particular, ingestion of foods rich in fat has been reported to modulate emotional states in humans and animal models.
- Animal studies have led the way in showing that specific strains of Bifidobacteria, Lactobacillus or Bacteroides can have positive effects on brain and behaviour, including evidence that certain bacteria can enhance cognitive processes and affect emotional learning.