It’s known that the omega-3 fatty acids docosahexaenoic acid (DHA) is crucial to brain function, vision and the regulation of inflammatory phenomena. It is also linked to reduced incidence of cancer. A team of researchers from University of Louvain (UCLouvain), have discovered the biochemical mechanism showing that other related fatty acids and DHA may slow the development of tumors, as published in Cell Metabolism.
This preliminary research showed that within a few days of exposure to DHA, tumor cells in acidosis literally gorge themselves on DHA but cannot store it correctly and literally poison themselves and die. Though the research is early, these scientists say that one should consume at least 250mg of DHA for reduced cancer risk.
This new research is based on 2016 findings from Olivier Feron’s UCLouvain team, who discovered that cells in an acidic microenvironment (acidosis) within tumors replace glucose with lipids as an energy source in order to multiply. This later led to findings in 2020 that these same cells are the most aggressive and acquire the ability to leave the original tumour to generate metastases. the most recent research, builds on this through a newly formed interdisciplinary team.
The interdisciplinary team first identified that acidotic tumour cells responded in diametrically opposite ways depending on the fatty acid they were absorbing. Within a few weeks, the results were both impressive and surprising. “We soon found that certain fatty acids stimulated the tumor cells while others killed them,” the researchers explained. DHA literally poisons them.
DHA May Slow the Development of Tumors
The poison acts on tumor cells via a phenomenon called ferroptosis, a type of cell death linked to the peroxidation of certain fatty acids. The greater the amount of unsaturated fatty acids in the cell, the greater the risk of their oxidation. Normally, in the acidic compartment within tumors, cells store these fatty acids in lipid droplets, a kind of bundle in which fatty acids are protected from oxidation. But in the presence of a large amount of DHA, the tumor cell is overwhelmed and cannot store the DHA, which oxidizes and leads to cell death.
This UCLouvain study shows the value of DHA in fighting cancer. “For an adult,” the UCLouvain researchers stated, “it’s recommended to consume at least 250 mg of DHA per day. But studies show that our diet provides on average only 50 to 100 mg per day. This is well below the minimum recommended intake.”
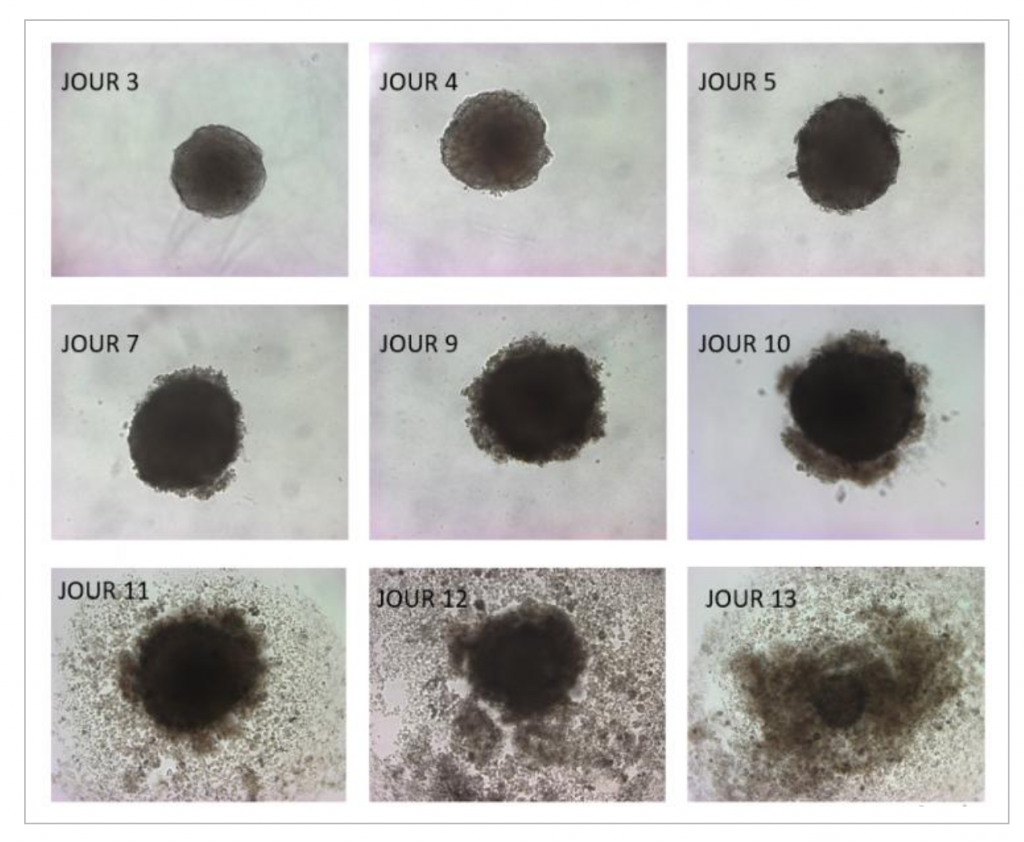
IMAGE OF CELL DEATH FROM DHA (Watch Time Lapse Here)
By using a lipid metabolism inhibitor that prevents the formation of lipid droplets, researchers were able to observe that this phenomenon is further amplified, which confirms the identified mechanism and opens the door to combined treatment possibilities.
For their study, UCLouvain researchers used a 3D tumor cell culture system, called spheroids. In the presence of DHA, spheroids first grow and then implode. The team also administered a DHA-enriched diet to mice with tumors. The result: tumor development was significantly slowed compared to that in mice on a conventional diet.
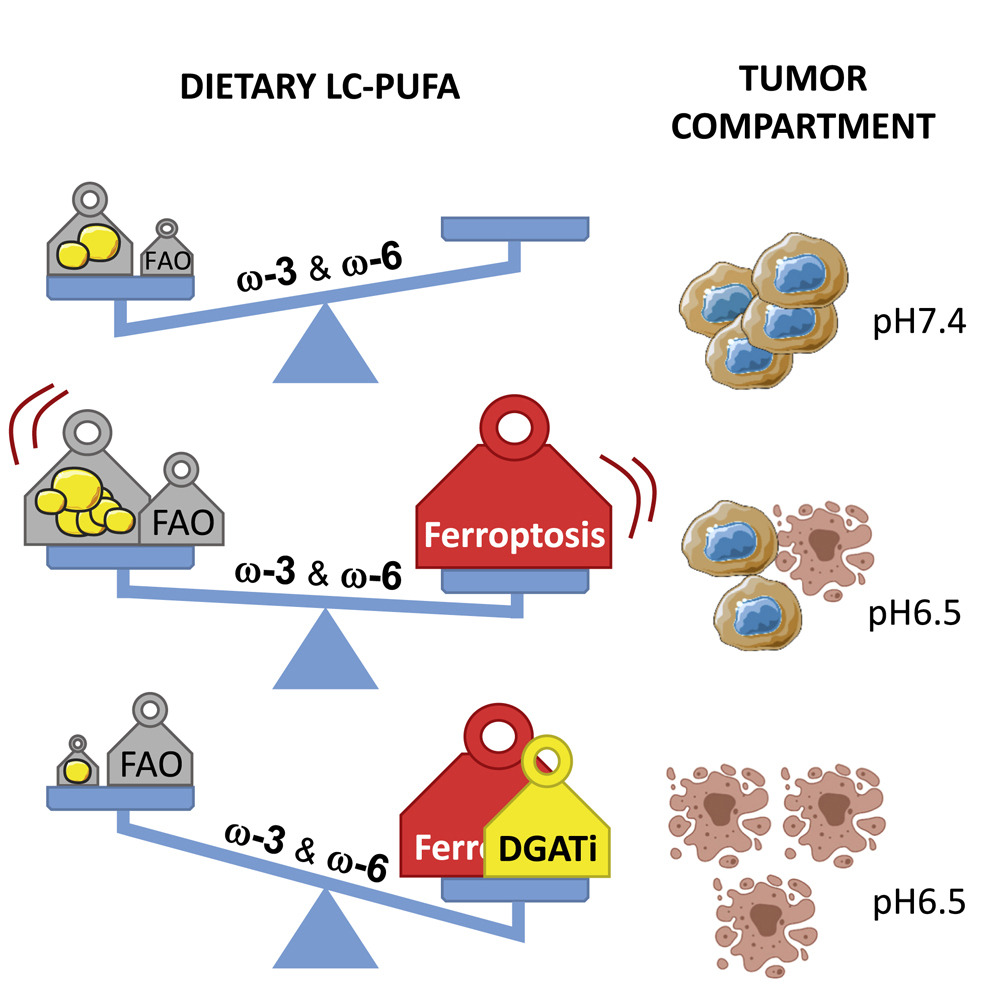
In summary (see below for diagram):
- Tumor acidosis promotes disease progression through a stimulation of fatty acid (FA) metabolism in cancer cells.
- n-3 and n-6 polyunsaturated FA (PUFA) selectively induced ferroptosis in cancer cells under ambient acidosis.
- When the cells reached excessive buffering capacity of triglyceride storage into lipid droplets, n-3 and n-6 PUFA peroxidation led to cytotoxic effects in proportion to the number of double bonds and even more so in the presence of diacylglycerolacyltransferase inhibitors (DGATi).
- Finally, an n-3 long-chain PUFA-rich diet significantly delayed mouse tumor growth when compared with a monounsaturated FA-rich diet, an effect further accentuated by administration of DGATi or ferroptosis inducers.
- The data points out that dietary PUFA as a selective adjuvant antitumor modality that may efficiently complement pharmacological approaches.