With one in 59 children diagnosed with autism, there is an urgent need to find the causes of the disorder. University of Central Florida (UCF) researchers are now a step closer to showing the link between the effect of Propionic acid (PPA), a short-chain fatty acid (SCFA) consumed during pregnancy and the effects on a fetus’ developing brain.
Today’s Practitioner recently reported on PPA as a trigger for insulin resistance (click here). The preservative PPA is used in baked goods, animal feeds, and artificial flavorings. This new study, though early in the process, offers clues about the effect of PPA on human neural stem cells (hNSCs) proliferation, differentiation and inflammation.
“PPA in the gut illustrates the interplay between the microbiome identity and dietary habits (nature versus nurture),” said UCF researchers.
Autism spectrum disorder (ASD) is characterized by glia over-proliferation, neuro-inflammation, perturbed neural circuitry, and gastrointestinal symptoms. The role of gut dysbiosis in ASD is an intriguing area of research that needs further elucidation.
Studies show that for individuals with ASD, there is a shift in the microbiome with elevated levels of Clostridia spp., Bacteriodetes, and Desulfovibrio spp.. These micro-organisms are known to be active fermenters of dietary carbohydrates and fibers leading to production of energy metabolism byproducts such as acetate (AC), propionate (PPA), and butyrate (BA).
Drs. Saleh Naser, Latifa Abdelli and UCF undergraduate research assistant Aseela Samsam identified the molecular changes that happen when neural stem cells are exposed to high levels of an acid commonly found in processed foods. The study published in Scientific Reports, a Nature journal, the UCF scientists discovered how high levels of Propionic Acid (PPA), used to increase the shelf life of packaged foods and inhibit mold in commercially processed cheese and bread, reduce the development of neurons in fetal brains.
Dr. Naser, who specializes in gastroenterology research at the College of Medicine’s Burnett School of Biomedical Sciences, began the study after reports showed that autistic children often suffer from gastric issues such as irritable bowel syndrome. He wondered about a possible link between the gut and the brain and began examining how the microbiome – or gut bacteria – differed between people with autism and those who do not have the condition.
“Studies have shown a higher level of PPA in stool samples from children with autism and the gut microbiome in autistic children is different,” Dr. Naser said. “I wanted to know what the underlying cause was.”
The researchers hypothesized that elevated PPA may tamper with neural cell plasticity and differentiation in vitro leading to gliosis, increased inflammatory profile, and disturbed neural connectivity, similar to ASD. In the lab, the scientists found exposing neural stem cells to excessive PPA damages brain cells in several ways.
- First, the acid disrupts the natural balance between brain cells by reducing the number of neurons and over-producing glial cells.
- While glial cells help develop and protect neuron function, too many glia cells disturb connectivity between neurons.
- They also cause inflammation, which has been noted in the brains of autistic children.
- Excessive amounts of the acid also shorten and damage pathways that neurons use to communicate with the rest of the body.
- The combination of reduced neurons and damaged pathways impede the brain’s ability to communicate, resulting in behaviors that are often found in children with autism, including repetitive behavior, mobility issues and inability to interact with others.
- PPA occurs naturally in the gut and a mother’s microbiome changes during pregnancy and can cause increases in the acid. But Drs. Naser and Abdelli said eating packaged foods containing the acid can further increase PPA in the woman’s gut, which then crosses to the fetus. See the figure below.
FIGURE 1
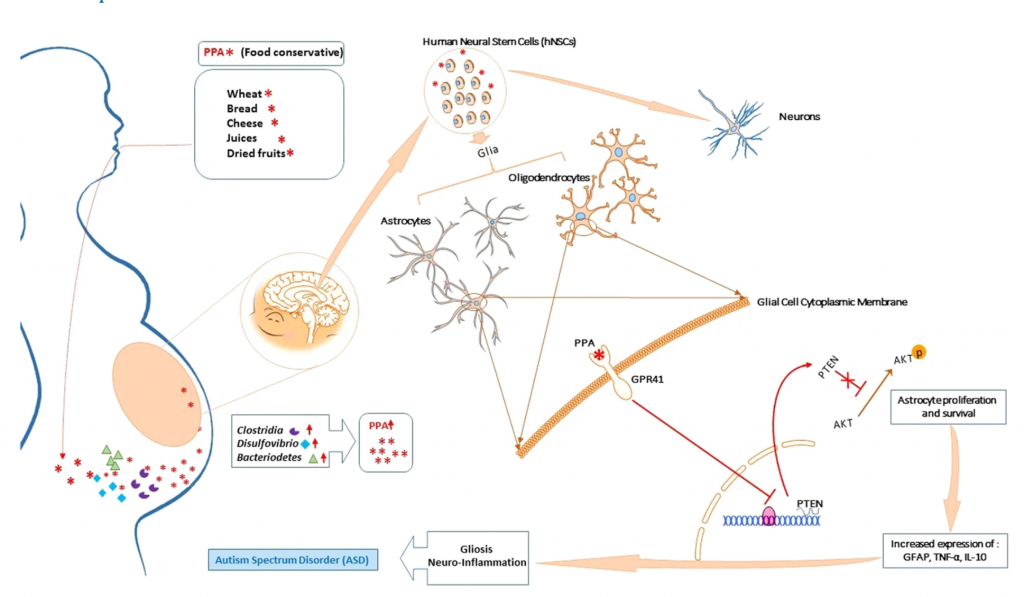
“This research is only the first step towards better understanding of Autism Spectrum Disorder,” the UCF scientists concluded. “But we have confidence we are on the right track to finally uncovering autism etiology.”
Overall, the data in this study suggest that microbiome shift in maternal gut leads to formation of by-product such as PPA which then interferes with neural patterning during the early stages of the fetus’ neural development. This favors glial progenitor cells proliferation and survival leading to increased inflammatory profile and perturbed neural architecture. The data further suggests that such process is achieved through modulation of PTEN/Akt pathway within the growing glial cells but not neurons.
Conclusion / The researchers conclude: “solving the conundrical etiology of ASD is critical for any future prevention or treatment strategies. There is no doubt that genetic polymorphisms and environmental triggers are both involved in ASD development or at least in ASD complications. Because of the fact that autistic individuals who undergo antibiotic treatment seem to demonstrate a provisory yet noticeable relief from GI symptoms and some ASD behavior amelioration, and they may benefit from fecal replacement as a method to restore their microbiota pool, there are good reasons to suggest that gut-brain axis is a potential culprit in ASD pathogenesis.”
Previous studies have proposed links between autism and environmental and genetic factors, but Drs. Naser and Abdelli say their study is the first to discover the molecular link between elevated levels of PPA, proliferation of glial cells, disturbed neural circuitry and autism.
More research needs to be done before drawing clinical conclusions. Next, the research team will attempt to validate its findings in mice models by seeing if a high PPA maternal diet causes autism in mice genetically predisposed to the condition. There is no cure for autism, which affects about 1 in 59 children, but the scientists hope their findings will advance studies for ways to prevent the disorder.